Find a clinical trial
Please use one or more of the field below to begin your search.
All clinical trials
-
Protocol number: 022-REDUCLN-002
Efficacy of the COronary SInus Reducer in Patients with Refractory Angina II (COSIRA-II)
-
Protocol number: 10445
Phase I Dose Escalation and Expansion Study of Tazemetostat in Combination With Topotecan and Pembrolizumab in Recurrent Small Cell Lung Cancer
-
Protocol number: 1150-101/PXL262601
A Phase 1/2, Open-label Study to Investigate the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of NDI-101150 Administered as Monotherapy or in Combination With Pembrolizumab in Patients With Solid Tumors
-
Protocol number: 1705018187-NCT04843566
Randomized controlled trial assessing transperineal prostate biopsy to reduce infection complications
-
Protocol number: 18H-MC-BDCV
A Phase 3, Parallel-Design, Open-Label, Randomized Controlled Study to Evaluate the Efficacy and Safety of LY3209590 as a Weekly Basal Insulin Compared to Insulin Glargine in Adults with Type 2 Diabetes on Multiple Daily Injections
-
Protocol number: 19-047-NCT04139902
Randomized Phase II Neoadjuvant Study of PD-1 Inhibitor Dostarlimab (TSR-042) vs. Combination of Tim-3 Inhibitor Cobolimab (TSR-022) and PD-1 Inhibitor Dostarlimab (TSR-042) in Resectable Stage III or Oligometastatic Stage IV Melanoma (Neo-MEL-T)
-
Protocol number: 2013-0496
A cross-over pilot study of the effect of amiloride compared to triamterene on proteinuria in patients with proteinuric kidney disease
-
Protocol number: 2014-0755
Identifying children with diabetes type 1 at high risk for cardiovascular disease
-
Protocol number: 2015-0017
A Randomized Trial Comparing the Outcomes for 2 Operations versus (greater than or equal to) 3 Operations in Infected Wounds Requiring Hospitalization
-
Protocol number: 2017-0385
Interobserver Variability in Grading Cervical Stenosis Using Sagittal Oblique Reformatted Images
-
Protocol number: 2017-0478
STEPs to Blood Pressure Reduction. A Randomized Clinical Trial of the Stroke Transitions, Education, and Prevention Clinic Versus Usual Care for Post-Stroke Blood Pressure Reduction
-
Protocol number: 2017-0700-NCT03685461
Intra- and Post-Operative Measures of Auditory Function
-
Protocol number: 2017-0961
MRI evaluation of recurrent disk herniation without post-contrast imaging using 3D T2 SPACE
-
Protocol number: 2017-1041-NCT03104517
CELLEBRATE: An Adaptive, Two-Stage, Double-Blind, Stratified, Randomized, Controlled Trial Comparing the Safety and Efficacy of AMDC-USR with Placebo in Female Subjects with Stress Urinary Incontinence
-
Protocol number: 2018-0191
Investigation of the Effects of Radioactive Iodine on the Salivary Glands
-
Protocol number: 2018-0439
A Randomized Trial of Proactive Outreach and Streamlined Genetic Education in BRCA Families
-
Protocol number: 2018-242
Outcomes Evaluation of the Medial Femoral Trochlea Flap for Reconstruction of the Scaphoid and Lunate
-
Protocol number: 20180244-OCEANA
A Double-blind, Randomized, Placebo-controlled, Multicenter Study Assessing the Impact of Olpasiran on Major Cardiovascular Events in Patients with Atherosclerotic Cardiovascular Disease and Elevated Lipoprotein (a)
-
Protocol number: 208090
A Phase III, randomized, multicenter, open-label, non-inferiority study evaluating the efficacy, safety and tolerability of switching to dolutegravir/lamivudine fixed dose combination in HIV-1 infected adults who are virologically suppressed
-
Protocol number: 209639-VH3810109
A Phase 2b Multicenter, Randomized, Open-Label Study Comparing the Efficacy, Safety, PK, and Tolerability of VH3810109, Administered Either Intravenously Or As A Subcutaneous Infusion with rHuPH20, in Combination with CAB LA to Standard of Care in Virologically Suppressed Adults Living with HIV
-
Protocol number: 21-02-348-NCT05155501
Pelvic fascia spARing radical prostatectomy TrIAL (PARTIAL)
-
Protocol number: 22-018-ITOG
22-018: Restoration of Radioiodine Uptake with Selpercatinib in RET Fusion-Positive Radioiodine-Refractory Thyroid Cancer: A Phase 2 Study Performed in Collaboration with the International Thyroid Oncology Group (ITOG)
-
Protocol number: 221AD304
A Study to Evaluate Safety and Tolerability of Aducanumab in Participants With Alzheimer's Disease Who Had Previously Participated in the Aducanumab Studies 221AD103, 221AD301, 221AD302 and 221AD205 (EMBARK)
-
Protocol number: 230LE303
A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of BIIB059 in Adult Participants with Active Systemic Lupus Erythematosus Receiving Background Nonbiologic Lupus Standard of Care
-
Protocol number: 5063
: Role of a CCK receptor antagonist proglumide in management of chronic pancreatitis
-
Protocol number: 61186372GIC2002-NCT05379595
A Phase 1b/2, Open-Label Study of Amivantamab Monotherapy and in Addition to Standard-of-Care Chemotherapy in Participants with Advanced or Metastatic Colorectal Cancer
-
Protocol number: 63733657ALZ2002
A Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicenter Study to Assess the Efficacy and Safety of JNJ-63733657, an Anti-tau Monoclonal Antibody, in Participants With Early Alzheimer's Disease
-
Protocol number: 64407564MMY3002-NCT05455320
A Phase 3 Randomized Study Comparing Talquetamab SC in Combination With Daratumumab SC and Pomalidomide (Tal-DP) or Talquetamab SC in Combination With Daratumumab SC (Tal-D) Versus Daratumumab SC, Pomalidomide and Dexamethasone (DPd), in Participants With Relapsed or Refractory Multiple Myeloma who Have Received at Least 1 Prior Line of Therapy
-
Protocol number: 68284528MMY4006-NCT05346835
Intermediate-Size Population Expanded Access Program (EAP) for Ciltacabtagene autoleucel (cilta-cel) Out-of-Specification (OOS) in patients with Multiple Myeloma
-
Protocol number: 718-CIH-201
A Randomized, Placebo-Controlled, Double-Blind Study to Evaluate the Effect of SAGE-718 on Cognitive Function in Participants with Huntington s Disease
-
Protocol number: 718-CIH-202
A 28-Day Randomized, Placebo-Controlled, Double-Blind, Parallel Groups and Normative Comparison Study to Evaluate the Effect of SAGE-718 on Functioning Capacity in Participants with Huntington's Disease
-
Protocol number: 718-CIH-301
PHASE 3 OPEN-LABEL SAFETY STUDY OF SAGE-718 IN PARTICIPANTS WITH HUNTINGTON'S DISEASE
-
Protocol number: A011801-COMPASSHER-NCT04457596
The COMPASSHER2 Trials (COMprehensive Use of Pathologic Response ASSessment to Optimize Therapy in HER2-Positive Breast Cancer): COMPASSHER2 Residual Disease (RD), A Double-Blinded, Phase III Randomized Trial of T-DM1 Compared with T-DM1 and Tucatinib
-
Protocol number: A012103
De-Escalation of Therapy in Early-Stage TNBC Patients Who Achieve pCR After Neoadjuvant Chemotherapy with Checkpoint Inhibitor Therapy"
-
Protocol number: A021806-NCT04340141
A Phase III Trial of Perioperative versus Adjuvant Chemotherapy for Resectable Pancreatic Cancer
-
Protocol number: A022104-JANUS
The Janus Rectal Cancer Trial: A Randomized Phase II Trial Testing the Efficacy of Triplet Versus Doublet Chemotherapy to Achieve Clinical Complete Response in Patients with Locally Advanced Rectal Cancer
-
Protocol number: A031704-PDIGREE-NCT0379316
A031704, PD-inhibitor (Nivolumab) and Ipilimumab followed by nivolumab vs. VEGF TKI cabozantinib with nivolumab in metastatic untreated REnal Cell CancEr [PDIGREE]
-
Protocol number: A051902-NCT04803201
A RANDOMIZED PHASE II STUDY OF CHO(E)P VS CC-486-CHO(E)P VS DUVELISIB-CHO(E)P IN PREVIOUSLY UNTREATED CD30 NEGATIVE PERIPHERAL T-CELL LYMPHOMAS
-
Protocol number: A081801-NCT04267848
Testing the Addition of a Type of Drug Called Immunotherapy to the Usual Chemotherapy Treatment for Non-small Cell Lung Cancer, ALCHEMIST Chemo-IO Study
-
Protocol number: A082002
A Randomized Phase II/III Trial of Modern Immunotherapy Based Systemic Therapy With or Without SBRT for PD-L1-Negative, Advanced Non-Small Cell Lung Cancer
-
Protocol number: A092104
A RANDOMIZED PHASE 2/3 STUDY OF OLAPARIB PLUS TEMOZOLOMIDE VERSUS INVESTIGATOR S CHOICE FOR THE TREATMENT OF PATIENTS WITH ADVANCED UTERINE LEIOMYOSARCOMA AFTER PROGRESSION ON PRIOR CHEMOTHERAPY
-
Protocol number: A092107
A RANDOMIZED PHASE 2 TRIAL WITH A SAFETY LEAD-IN TO EVALUATE PALBOCICLIB VERSUS PALBOCICLIB AND CEMIPLIMAB FOR THE TREATMENT OF ADVANCED DEDIFFERENTIATED LIPOSARCOMA
-
Protocol number: A1334-02
A Phase 1b Randomized, Double-Blind, Placebo-Controlled, Multiple-Ascending Dose Study to Evaluate the Safety, Pharmacokinetics, and Pharmacodynamics of ACE-1334 Plus Standard of Care in Participants with Systemic Sclerosis
-
Protocol number: A151216-ALCHEMIST-NCT02194738
Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST)
-
Protocol number: A211801-BRCA-P-NCT04711109
A Randomized, Double-Blind, Placebo-Controlled, Multi-Center, International Phase 3 Study to determine the Preventive Effect of Denosumab on Breast Cancer in Women carrying a BRCA1 Germline Mutation
-
Protocol number: A212102-MULTICANCER-NCT0533406
Blinded Reference Set For Multicancer Early Detection Blood Tests
-
Protocol number: A22-301
A Single-arm, Multicenter Study to Assess the Efficacy, Safety, and Tolerability of Ropeginterferon alfa-2b-njft (P1101) in Adult Patients with Essential Thrombocythemia
-
Protocol number: AALL1731-NCT03914625
A Phase 3 Trial Investigating Blinatumomab (IND# 117467, NSC# 765986) in Combination with Chemotherapy in Patients with Newly Diagnosed Standard Risk or Down syndrome B-Lymphoblastic Leukemia (B-ALL) and the Treatment of Patients with Localized B-Lymphoblastic Lymphoma (B-LLy)
-
Protocol number: AALL1732-NCT03959085
A Phase 3 Randomized Trial of Inotuzumab Ozogamicin (IND#:133494, NSC#: 772518) for Newly Diagnosed High-Risk B-ALL; Risk-Adapted Post-Induction Therapy for High-Risk B-ALL, Mixed Phenotype Acute Leukemia, and Disseminated B-LLy
-
Protocol number: ABBVIEM23-072
A Phase 4, Multicenter, Open-Label Study to Evaluate the Safety, Tolerability, and Efficacy of the Concomitant Use of Ubrogepant for the Acute Treatment of Migraine in Subjects Taking Atogepant for the Preventive Treatment of Episodic Migraine
-
Protocol number: ACT17209
A multicenter, open-label, Phase IIb study to evaluate the efficacy, safety and pharmacokinetics of rilzabrutinib in patients with warm autoimmune hemolytic anemia
-
Protocol number: ACTNOW
Pragmatic, Randomized, Blinded Trial to Shorten Pharmacologic Treatment of Newborns with Neonatal Opioid Withdrawal Syndrome (NOWS)
-
Protocol number: ACURATE-NCT03735667
Transcatheter Replacement of Stenotic Aortic Valve through Implantation of ACURATE in Subjects InDicatEd for TAVR
-
Protocol number: ADMIRE-BWI201910
Assessment of Safety and Effectiveness in Treatment Management of Atrial Fibrillation with the BWI IRE Ablation System
-
Protocol number: ADNI3-NCT02854033
Alzheimers Disease Neuroimaging Initiative 3 (ADNI3)
-
Protocol number: ADVANCE-STUDY
EndurAnt Stent Graft system vs ExcluDer endoprothesis: a global, prospectiVe, rANdomized Clinical trial in sac rEgression (ADVANCE Study)
-
Protocol number: AFFINITY-NCT03222973
A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study in Subjects With Relapsing Multiple Sclerosis to Evaluate the Efficacy and Safety of BIIB033 as an Add-On Therapy to Anti-Inflammatory Disease-Modifying Therapies
-
Protocol number: AFT-50-NCT04486352
A Phase IB/II Multi-Cohort Study of Targeted Agents With Atezolizumab for Patients With Recurrent or Persistent Endometrial Cancer
-
Protocol number: AGCT1531-NCT03067181
A Phase 3 Study of Active Surveillance for Low Risk and a Randomized Trial of Carboplatin vs. Cisplatin for Standard Risk Pediatric and Adult Patients With Germ Cell Tumors
-
Protocol number: AGCT1532-NCT02582697
Phase 3 Accelerated BEP: A Randomised Phase 3 Trial of Accelerated Versus Standard BEP Chemotherapy for Patients With Intermediate and Poor-risk Metastatic Germ Cell Tumours
-
Protocol number: AGT-HC168
Phase I Study to Evaluate the Safety of Genetically Modified, Autologous T Cells in Participants with HIV that is Well-Controlled on Antiretroviral Therapy
-
Protocol number: AGT103-T-LTFU
A Long-Term Follow-Up Study of Participants Treated with the LentiviralBased Genetically Modified, Autologous Cell Product, AGT103-T
-
Protocol number: AHEAD3-45
AHEAD 3-45 Study: A Placebo-Controlled, Double-Blind, Parallel-Treatment Arm, 216 Week Study to Evaluate Efficacy and Safety of Treatment With BAN2401 in Subjects With Preclinical Alzheimer s Disease and Elevated Amyloid (A45 Trial) and in Subjects With Early Preclinical Alzheimer s Disease and Intermediate Amyloid (A3 Trial)
-
Protocol number: AHOD2131-NCT05675410
A Randomized Phase 3 Interim Response Adapted Trial Comparing Standard Therapy With Immuno-oncology Therapy for Children and Adults With Newly Diagnosed Stage I and II Classic Hodgkin Lymphoma
-
Protocol number: AIBERRY-STUDY00003473
Developing an A.I. Platform to Detect Depression Predict depression at higher degree of accuracy Implement the Aiberry platform in a live environment
-
Protocol number: AIMPOWER-NCT04191330
Artificial Intelligence Mobile Health Trial Of A Digital Platform To Optimize GDMT Using Wearable Sensors
-
Protocol number: AIS-D04
A PHASE 1B, OPEN-LABEL STUDY TO ASSESS THE SAFETY, EFFICACY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF ALPN-303 IN SUBJECTS WITH AUTOIMMUNE CYTOPENIAS (RUBY-4)
-
Protocol number: AL002-2
A PHASE 2 RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO EVALUATE THE EFFICACY AND SAFETY OF AL002 IN PARTICIPANTS WITH EARLY ALZHEIMER S DISEASE
-
Protocol number: ALIGN-AR-NCT04415047
A Study to Assess Safety and Probable Benefit of the Transfemoral JenaValve Pericardial TAVR System in the Treatment of High Surgical Risk Patients with Symptomatic, Severe Aortic Regurgitation (AR)
-
Protocol number: ALLIANCE-AVIV
Safety and Effectiveness of Balloon-Expandable Bioprosthetic SAPIEN X4 Transcatheter Heart Valve in Failing Aortic Surgical Bioprosthetic Valves
-
Protocol number: ALLIANCE-NCT05172960
Safety and Effectiveness of Balloon-Expandable Bioprosthetic SAPIEN X4 Transcatheter Heart Valve
-
Protocol number: ALLO-316-101-NCT04696731
A PHASE 1 MULTICENTER STUDY EVALUATING THE SAFETY AND EFFICACY OF ALLO-316 FOLLOWING ALLO-647 CONTAINING CONDITIONING REGIMEN IN SUBJECTS WITH ADVANCED OR METASTATIC CLEAR CELL RENAL CELL CARCINOMA (ccRCC)
-
Protocol number: ALTE1631-NCT03223753
A Randomized Web-based Physical Activity Intervention among Children and Adolescents with Cancer
-
Protocol number: ALXN1720-MG-301-NCT05556096
A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel, Multicenter Study to Evaluate the Safety and Efficacy of ALXN1720 in Adults with Generalized Myasthenia Gravis
-
Protocol number: ALXN2220-ATTRCM-301/NCT0618393
DepleTTR-CM A Phase 3, Randomized, Double-blind, Placebo-controlled, Multicenter Study to Evaluate the Efficacy and Safety of Amyloid Depleter ALXN2220 in Adult Participants with Transthyretin Amyloid Cardiomyopathy (ATTR-CM)
-
Protocol number: AML-PROSPECTIVE-STUDY00000298
A Pilot Study to Evaluate Physical Function and Fatigue in Patients with Acute Myeloid Leukemia Single Center Prospective Cohort Study
-
Protocol number: AML-RETROSPECTIVE
A center-based chart review study to assess treatment outcomes of venetoclax for the treatment of acute myeloid leukemia (AML)
-
Protocol number: AMPLATZER-PFO-NCT03309332
AMPLATZER PFO Occluder Post Approval Study (PFO PAS)
-
Protocol number: AMPRELOXETINE-0197-CYPRESS
A Phase 3, Multi-center, Randomized Withdrawal and Long-Term Extension Study of Ampreloxetine for the Treatment of Symptomatic Neurogenic Orthostatic Hypotension in Participants with Multiple System Atrophy
-
Protocol number: ANHL1931-NCT04759586
Nivolumab in Combination With Chemo-Immunotherapy for the Treatment of Newly Diagnosed Primary Mediastinal B-Cell Lymphoma
-
Protocol number: AP-PA02-201
A Phase 2, Multi-Center, Double-Blind, Randomized, Placebo Controlled Study to Evaluate the Safety, Phage Kinetics, and Efficacy of Inhaled AP-PA02 Multi-Phage Therapeutic in Subjects with Non-Cystic Fibrosis Bronchiectasis and Chronic Pulmonary Pseudomonas aeruginosa Infection
-
Protocol number: APEC14B1
APEC14B1: Project:EveryChild A Registry, Eligibility Screening, Biology and Outcome Study
-
Protocol number: APEC1621SC-NCT03155620
NCI-COG Pediatric MATCH (Molecular Analysis for Therapy Choice) Screening Protocol
-
Protocol number: APOL-1
Integrating a Culturally Competent APOL1 Genetic Testing Program into Living Donor Evaluation
-
Protocol number: APOLLO-NCT03242642
Transcatheter Mitral Valve Replacement with the Medtronic Intrepid TMVR System in patients with severe symptomatic mitral regurgitation APOLLO Trial
-
Protocol number: APT-DFI
A Phase 2b Randomized, Parallel Group, Double-Blind, Placebo-Controlled, Repeat Dose, MultiSite Study Investigating the Safety, Tolerability, and Efficacy of Personalized Phage Treatment and Standard of Care Antimicrobials for Subjects with Diabetic Foot Osteomyelitis due to S. aureus
-
Protocol number: AREN1921-NCT04322318
Treatment of Newly Diagnosed Diffuse Anaplastic Wilms Tumors(DAWT)and Relapsed Favorable Histology Wilms Tumors(FHWT)
-
Protocol number: ARGX-113-2005
A Phase 3, Multicenter, Open-Label, Long-Term Trial to Evaluate the Safety and Efficacy of Efgartigimod (ARGX-113) PH20 Subcutaneous in Adult Patients With Primary Immune Thrombocytopenia
-
Protocol number: ARISEII-NCT05800743
Evaluation of the GORE® Ascending Stent Graft (ASG device) in the Treatment of Lesions of the Ascending Aorta
-
Protocol number: ARST2031-NCT04994132
A Randomized Phase 3 Trial of Vinorelbine, Dactinomycin, and Cyclophosphamide (VINO-AC) Plus Maintenance Chemotherapy with Vinorelbine and Oral Cyclophosphamide (VINO-CPO) vs Vincristine, Dactinomycin and Cyclophosphamide (VAC) plus VINO-CPOMaintenance in Patients with High Risk Rhabdomyosarcoma (HR-RMS)
-
Protocol number: ASELL-SPECIAL-POPULATIONS
Clinical Validation of the CytoRADx System as a Biodosimeter for Special Human Populations
-
Protocol number: ASPIRE-NCT03907046-GUMC
Anticoagulation in Intracerebral Hemorrhage (ICH) Survivors for Stroke Prevention and Recovery
-
Protocol number: ATA129-EBV-302-NCT03394365
Multicenter, Open Label, Phase 3 Study of Tabelecleucel for Solid Organ or Allogeneic Hematopoietic Cell Transplant Subjects With Epstein-Barr Virus-Associated Post-Transplant Lymphoproliferative Disease After Failure of Rituximab or Rituximab and Chemotherapy
-
Protocol number: ATN-106
A multicentre, prospective, open-label, uncontrolled Phase 3 study to assess the efficacy, safety and pharmacokinetics of Atenativ in patients with congenital antithrombin deficiency undergoing surgery or delivery
-
Protocol number: ATX-MAP-001-NCT03517917
Prospective collection of donor tissue and whole blood or leukapheresis product from patients with solid tumors to enable development of methods for the manufacturing of clonal neoantigen T cell products (cNeT).
-
Protocol number: AURORAUS-NCT03737695
AURORA US: PROSPECTIVE BIOSPECIMEN REPOSITORY IN METASTATIC BREAST CANCER
-
Protocol number: AVENTUS
Assessing the Safety and Efficacy of the treatment for Acute Pulmonary Embolism using the Inquis Medical Aventus Thrombectomy System.
-
Protocol number: AXS-07-304
An Open-Label, Multiple-Dose Evaluation of the Efficacy and Safety of AXS-07 (meloxicam and rizatriptan) for the Acute Treatment of Migraine in Adults with a Prior Inadequate Response to an Oral CGRP Inhibitor
-
Protocol number: B7981040
A PHASE 3 RANDOMIZED, DOUBLE-BLIND, 52-WEEK PLACEBOCONTROLLED, MULTI-CENTER STUDY INVESTIGATING THE EFFICACY, SAFETY, AND TOLERABILITY OF RITLECITINIB IN ADULT AND ADOLESCENT PARTICIPANTS WITH NON SEGMENTAL VITILIGO
-
Protocol number: BAN2401-G000-301
A Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study With an Open-Label Extension Phase to Confirm Safety and Efficacy of BAN2401 in Subjects With Early Alzheimer s Disease.
-
Protocol number: BBI-20201001-NCT04278144
Phase 1 Study of BDC-1001 as a Single Agent and in Combination with Pembrolizumab in Patients with Advanced and HER2- Expressing Solid Tumors
-
Protocol number: BDPI-20-004-NCT05711316
The BD Liverty TIPS Stent Graft Study - A Global, Prospective, Multi-Center, Single-Arm Clinical Study for the Treatment of Complications from Portal Hypertension (ARCH)
-
Protocol number: BDTX-4933-101-NCT05786924
A Phase 1, Open-label Study of BDTX-4933 in Patients With BRAF and Other Select RAS/MAPK Mutation-Positive Neoplasms
-
Protocol number: BFR-HITT-NCT06214494
Effects of Blood Flow Restricted Treadmill Training on Independently Ambulating Chronic Ischemic CVA Survivors: A Pilot Study
-
Protocol number: BGB-11417-201-NCT05471843
A Single-Arm, Open-Label, Multicenter Phase 2 Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Bcl-2 Inhibitor BGB-11417 in Patients With Relapsed or Refractory Mantle Cell Lymphoma
-
Protocol number: BHV3500-203
BHV3500-203: Phase 2/3: Double-Blind, Randomized, Placebo Controlled, Safety and Efficacy Trial of Vazegepant (BHV-3500) Intranasal (IN) for Hospitalized Patients with COVID-19 Requiring Supplemental Oxygen
-
Protocol number: BI-1479-0001-NCT04886804
An open label, Phase I dose escalation trial, with dose confirmation and expansion, of BI 1810631 as monotherapy in patients with advanced or metastatic solid tumors with HER2 aberrations
-
Protocol number: BI1305-0023
A double blind, randomized, placebo-controlled trial evaluating the efficacy and safety of BI 1015550 over at least 52 weeks in patients with Progressive Fibrosing Interstitial Lung Diseases (PF-ILDs)
-
Protocol number: BIO-CONDUCT-NCT05251363
BIOTRONIK Conduction System Pacing with the Solia Lead
-
Protocol number: BIODESIX
A Multicenter, Randomized Controlled Trial, Prospectively Evaluating the Clinical Utility of the Nodify XL2 Proteomic Classifier in Incidentally Discovered Low to Moderate Risk Lung Nodules
-
Protocol number: BIOGEN-275AS101-ALSPIRE
A Phase 1/2 Multiple-Ascending-Dose Study With a Long-Term Open-Label Extension to Assess the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Effect on Disease Progression of BIIB105 Administered Intrathecally to Adults With Amyotrophic Lateral Sclerosis With or Without Poly-CAG Expansion in the Ataxin-2 Gene
-
Protocol number: BK-JM-201
A Randomized, Double-Blind, Placebo-Controlled, Two-Part Study in Parkinson’s Disease Patients With Dyskinesia to Assess the Efficacy and Safety/Tolerability of Fixed Dose Combinations of JM-010 and its Individual Components
-
Protocol number: BLU-451-1101
PHASE 1/2 STUDY OF BLU-451 IN ADVANCED CANCERS WITH EGFR EXON20 INSERTION MUTATIONS
-
Protocol number: BMTCTN1903-NCT04975698
Administration of HIV-specific T cells to HIV+ Patients Receiving High Dose Chemotherapy Followed by Autologous Stem Cell Rescue -Auto-RESIST
-
Protocol number: BN42489
A PHASE II, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, DOSE-FINDING STUDY TO EVALUATE THE SAFETY, BIOMARKERS, AND EFFICACY OF TOMINERSEN IN INDIVIDUALS WITH PRODROMAL AND EARLY MANIFEST HUNTINGTON'S DISEASE
-
Protocol number: BOLT-NCT05003843
BOLT: A Prospective, Multicenter Study of Patients with Deep Vein Thrombosis to Evaluate the Safety and Efficacy of the Indigo® Aspiration System
-
Protocol number: BP-LVAD
Blood Pressure and Outcomes in Contemporary Left Ventricular Assist Device Recipients
-
Protocol number: BP44382
AN OPEN-LABEL, MULTICENTER PHASE I STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, PHARMACODYNAMICS, AND PRELIMINARY ANTI-TUMOR ACTIVITY OF RO7616789 IN PARTICIPANTS WITH ADVANCED SMALL CELL LUNG CANCER AND OTHER NEUROENDOCRINE CARCINOMAS
-
Protocol number: BRIDGES-NCT05448560
BRidging Information Divides and Gaps for Equity in Survivorship (BRIDGES)
-
Protocol number: BURN-RADIATION
A Systems Biology Approach to Radiation Biodosimetry and the Host- Environment Interaction: Applications to Mass Casualty Triage in the Polytrauma Patient.
-
Protocol number: C-14
CORRECT-MRD II: SECOND COLORECTAL CANCER CLINICAL VALIDATION STUDY TO PREDICT RECURRENCE USING A CIRCULATING TUMOR DNA ASSAY TO DETECT MINIMAL RESIDUAL DISEASE
-
Protocol number: C-800-23-NCT05529316
A MULTICOHORT, OPEN LABEL, PHASE 2 STUDY OF BOTENSILIMAB (AGEN1181) FOR TREATMENT OF ADVANCED MELANOMA REFRACTORY TO PRIOR CHECKPOINT INHIBITOR THERAPY
-
Protocol number: C3300
A Phase 2 Randomized, Double-blind, Placebo-controlled, Adaptive Study to Evaluate the Efficacy, Safety, and Tolerability of 2 Dose Levels of IW-3300 Administered Rectally for 12 Weeks to Treat Bladder Pain in Subjects with Interstitial Cystitis/Bladder Pain Syndrome
-
Protocol number: C3651011
A PHASE 2, DOUBLE-BLIND, RANDOMIZED, PLACEBO-CONTROLLED, 4-ARM STUDY TO INVESTIGATE SYMPTOMS, FUNCTION, HEALTH-RELATED QUALITY OF LIFE AND SAFETY WITH REPEATED SUBCUTANEOUS ADMINISTRATION OF PONSEGROMAB VERSUS PLACEBO IN ADULT PARTICIPANTS WITH HEART FAILURE
-
Protocol number: C4221015-NCT04607421
AN OPEN-LABEL, MULTICENTER, RANDOMIZED PHASE 3 STUDY OF FIRST-LINE ENCORAFENIB PLUS CETUXIMAB WITH OR WITHOUT CHEMOTHERAPY VERSUS STANDARD OF CARE THERAPY WITH A SAFETY LEAD-IN OF ENCORAFENIB AND CETUXIMAB PLUS CHEMOTHERAPY IN PARTICIPANTS WITH METASTATIC BRAF V600E-MUTANT COLORECTAL CANCER
-
Protocol number: C4391022-PF-07220060
AN INTERVENTIONAL, OPEN-LABEL, RANDOMIZED, MULTICENTER PHASE 3 STUDY OF PF-07220060 PLUS FULVESTRANT COMPARED TO INVESTIGATOR’S CHOICE OF THERAPY IN PARTICIPANTS OVER 18 YEARS OF AGE WITH HORMONE RECEPTOR-POSITIVE, HER2-NEGATIVE ADVANCED/METASTATIC BREAST CANCER WHOSE DISEASE PROGRESSED AFTER PRIOR CDK 4/6 INHIBITOR BASED THERAPY
-
Protocol number: C4671034
A Study to Learn About the Study Medicines Called Nirmatrelvir/Ritonavir in People at Least 12 Years of Age With COVID-19 Who Are Immunocompromised
-
Protocol number: CA209-548-NCT02667587
A Randomized Phase 3 Single Blind Study of Temozolomide Plus Radiation Therapy Combined With Nivolumab or Placebo in Newly Diagnosed Adult Subjects With MGMT-Methylated (Tumor O6-methylguanine DNA Methyltransferase) Glioblastoma
-
Protocol number: CA209498
A Randomized Phase 3 Open Label Study of Nivolumab vs Temozolomide Each in Combination with Radiation Therapy in Newly Diagnosed Adult Subjects with Unmethylated MGMT (tumor O-6-methylguanine DNA methyltransferase) Glioblastoma
-
Protocol number: CA224-098-NCT05002569
A Phase 3, Randomized, Double-blind Study of Adjuvant Immunotherapy With Nivolumab + Relatlimab Fixed-dose Combination Versus Nivolumab Monotherapy After Complete Resection of Stage III-IV Melanoma
-
Protocol number: CA224106-NCT05337137
A Phase 1/2, Safety Confirmation and Double-blind, Placebo-controlled, Randomized Study of Relatlimab in Combination with Nivolumab and Bevacizumab in Treatment-naive Advanced/Metastatic Hepatocellular Carcinoma
-
Protocol number: CA42481
A Phase II, Open-Label, Randomized, Multicenter Study To Investigate the Pharmacodynamics, Pharmacokinetics, Safety and efficacy of 8mg/kg or 4mg/kg Intravenous Tocilizumab in Patients with moderate to severe COVID-19 Pneumonia
-
Protocol number: CAAA601A42101
A Phase Ib Dose Finding Study Assessing Safety and Activity of [177Lu]Lu-DOTA-TATE in Newly Diagnosed Extensive Stage Small Cell Lung Cancer (ES-SCLC) in Combination with Carboplatin, Etoposide and Tislelizumab in Induction and with Tislelizumab in Maintenance Treatment Phase
-
Protocol number: CAD-DECISIONAID
Shared Decision Making in the Prevention and Management of Cardiovascular Disease
-
Protocol number: CAM5776-NCT04741009
A Pre-market, Open-label Feasibility, Prospective, Single-arm Multicenter Feasibility Investigation of Hearing Performance Using the CI632 in Adults With Low-frequency Residual Hearing.
-
Protocol number: CAPTIVA-NCT05047172
CAPTIVA is a two-stage Phase III trial randomizing subjects with stroke attributed to 70-99% intracranial atherosclerotic stenosis (sICAS) to: 1) ticagrelor + aspirin 2) low dose rivaroxaban + aspirin 3) clopidogrel + aspirin The primary goal of the trial is to determine if the experimental arm(s) (rivaroxaban or ticagrelor or both) are superior to the clopidogrel arm for lowering the 1-year rate of the primary endpoint (ischemic stroke, intracerebral hemorrhage (ICH), or vascular death). The first stage of the trial is concluded by an interim safety analysis intended to identify an excess of parenchymal intracerebral hemorrhage (ICH) or non-ICH major hemorrhage with ticagrelor + aspirin or low dose rivaroxaban + aspirin that could lead to an early termination of one or both of those arms. The second stage of the trial will determine if the experimental arm(s) (rivaroxaban or ticagrelor or both) that progress to stage 2 are superior to the clopidogrel arm for lowering the 1-year rate of the primary endpoint (ischemic stroke, intracerebral hemorrhage (ICH), or vascular death). An exploratory aim is to estimate the impact of CYP2C19 loss-of-function (LOF) carrier status on any benefit that the ticagrelor or low dose rivaroxaban arms may have in lowering the primary endpoint compared with the clopidogrel arm.
-
Protocol number: CARDIOL-100-102
Impact of CardiolRxTM on Myocardial Recovery in Acute Myocarditis
-
Protocol number: CARDUS-HANDHELD
Handheld Cardiac Ultrasound in Resident Assessment of Left Ventricular Ejection Fraction in Outpatients
-
Protocol number: CARILLON-NCT03142152
Assessment of the Carillon® Mitral Contour System® in Treating Functional Mitral Regurgitation Associated With Heart Failure - The CARILLON Trial
-
Protocol number: CATALYST-1073-310
Study of Hypercortisolism in Patients with Difficult to Control Type 2 Diabetes Despite Receiving Standard-of-Care Therapies: Prevalence and Treatment with Korlym® (Mifepristone) (CATALYST)
-
Protocol number: CATALYST-1073-310-GSM
Study of Hypercortisolism in Patients with Difficult to Control Type 2 Diabetes Despite Receiving Standard-of-Care Therapies: Prevalence and Treatment with Korlym® (Mifepristone) (CATALYST)
-
Protocol number: CATALYST-NCT04226547
Clinical trial of atrial fibrillation patients comparing left atrial appendage occlusion therapy to non-vitamin K antagonist oral anticoagulants
-
Protocol number: CATHBUDDY
PTC-006 CathBuddy, Inc. HLD Validation Through In-Use Testing Protocol
-
Protocol number: CC-94676-PCA-001
A PHASE 1, MULTI-CENTER, OPEN-LABEL, DOSE-FINDING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF CC-94676 IN SUBJECTS WITH METASTATIC CASTRATION-RESISTANT PROSTATE CANCER
-
Protocol number: CERCLAGEEFS-NCT03929913
NHLBI DIR Transcatheter Mitral Cerclage Annuloplasty early feasibility study
-
Protocol number: CGT9486-21-301-PEAK
A Phase 3 Randomized, Open-Label, Multicenter Clinical Study of CGT9486+Sunitinib vs. Sunitinib in Subjects With Locally Advanced, Unresectable, or Metastatic Gastrointestinal Stromal Tumors (PEAK)
-
Protocol number: CHAMPION-AF
WATCHMAN FLX versus NOAC for EMbolic ProtectION in the Management of Patients with Non-Valvular Atrial Fibrillation
-
Protocol number: CHIMES-NCT04377555
AN OPEN-LABEL, MULTICENTER STUDY TO ASSESS DISEASE ACTIVITY AND BIOMARKERS OF NEURONAL DAMAGE IN MINORITY PATIENTS WITH RELAPSING MULTIPLE SCLEROSIS RECEIVING TREATMENT WITH OCRELIZUMAB
-
Protocol number: CL-SBP-101-04-NCT05254171
A Randomized, Double-Blind, Placebo-Controlled Study of Nab-Paclitaxel and Gemcitabine With Or Without SBP-101 in Subjects Previously Untreated for Metastatic Pancreatic Ductal Adenocarcinoma
-
Protocol number: CLASP-IID-NCT03706833
Edwards PASCAL TrAnScatheter Mitral Valve RePair System Pivotal Clinical Trial (CLASP IID): A prospective, multicenter, randomized, controlled pivotal trial to evaluate the safety and effectiveness of transcatheter mitral valve repair with the Edwards PASCAL Transcatheter Mitral Valve Repair System compared to Abbott MitraClip in patients with degenerative mitral regurgitation (DMR).
-
Protocol number: CLIN-52120-463
A Phase III, Randomised, Double-blind, Placebo-controlled, Multicentre, Parallel-group Study with Extension Phase to Evaluate the Efficacy and Safety of Dysport® for the Prevention of Chronic Migraine in Adult Participants
-
Protocol number: CLIN-52120-464
A Phase III, Randomised, Double-blind, Placebo-controlled, Multicentre, Parallel-group Study with Extension Phase to Evaluate the Efficacy and Safety of Dysport® for the Prevention of Episodic Migraine in Adult Participants
-
Protocol number: CLNP023F12301
A multicenter, single-arm, open label trial to evaluate efficacy and safety of oral, twice daily LNP023 in adult aHUS patients who are naive to complement inhibitor therapy
-
Protocol number: CLR1806-NCT03655236
A PHASE 2, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY OF K0706 IN SUBJECTS WITH EARLY PARKINSON S DISEASE
-
Protocol number: CO-US-979-6770-NCT06248515
A Phase II Trial of Sacituzumab Govitecan-Hziy in Patients with Advanced Thymic Epithelial Tumors
-
Protocol number: COE-002-1116
Caris Molecular Intelligence® and Caris Centers of Excellence for Precision Medicine NetworkTM Retrospective Outcomes-Associated Database
-
Protocol number: COG0203
Synaptic Therapy Alzheimer's Research Trial (START): A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial to Evaluate the Safety and Efficacy of CT1812 in Early Alzheimer's Disease over 18 Months
-
Protocol number: COLOGUARD
A Real World Study of Patients Under the Age of 50 Screened for Colorectal Cancer (CRC) A Real World Study of Patients Under the Age of 50 Screened for Colorectal Cancer (CRC) Using Cologuard® in the U.S. Tidal
-
Protocol number: COLOPLAST-INTIBIA
A Prospective, Randomized Clinical Trial Evaluating INTIBIA, an Investigational Implantable Tibial Nerve Stimulator, Through 24-Months.
-
Protocol number: COMB157GUS10
AGNOS: An 18-month, Open-label, Multi-Center Study to Assess the Effect of Ofatumumab 20mg SC Monthly in Treatment Naïve, Very Early Relapsing Remitting Multiple Sclerosis Patients Benchmarked Against Healthy Controls on Select Outcomes.
-
Protocol number: CONFIDES-NCT04935177
A Phase III Randomized Controlled Trial to Evaluate 1-Year eGFR in Highly Sensitized (cPRA Greater than or equal to 99.9%) Deceased Donor Kidney Transplant Recipients, Following Imlifidase-Inactivation of HLA Antibodies Prior to Transplantation
-
Protocol number: CONFORM-NCT05147792
The CONFORM Pivotal Trial; An Evaluation of the Safety and Effectiveness of the Conformal CLAAS System for Left Atrial Appendage Occlusion
-
Protocol number: CONNECT-HF-NCT03035474
CARE OPTIMIZATION THROUGH PATIENT AND HOSPITAL ENGAGEMENT CLINICAL TRIAL FOR HEART FAILURE CONNECT-HF
-
Protocol number: CONVERGEPAS
Post-Approval Study Protocol For Hybrid Convergent Of Epicardial RF Ablation And Endocardial Ablation For The Treatment Of Symptomatic Long-standing Persistent AF
-
Protocol number: CORCINCH-HF
Randomized Clinical Evaluation of the AccuCinch® Ventricular Repair System in Patients who Present with Symptomatic Heart Failure with Reduced Ejection Fraction (HFrEF)
-
Protocol number: COREVITASSPHERES-NCT04886492
CorEvitas SPHERES (Synergy of Prospective Health & Experimental Research for Emerging Solutions) Registry for Neuromyelitis Optica Spectrum Disorder (NMOSD)
-
Protocol number: COREVNS-NCT03529045
Comprehensive Outcomes Registry in Subjects with Epilspsy Treated with Vagus Nerve Stimulation Therapy
-
Protocol number: CORMATRIX-NCT02397668
CORMATRIX® COR TRICUSPID ECM® VALVE REPLACEMENT SAFETY AND EARLY FEASIBILITY STUDY
-
Protocol number: COVID-CALYPSO
COVID-19 associated Lymphopenia Pathogenesis Study in Blood
-
Protocol number: COVID-REP0321
Reparixin 1200 mg three times a day as add-on therapy to standard of care to limit disease progression in hospitalised adult patients with COVID-19. A multinational, multicentre, randomised, doubleblinded, placebo-controlled, parallel-group phase III trial.
-
Protocol number: CT-868
A Phase 2, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of CT-868 Administered for 16 Weeks to Overweight and Obese Adult Participants with Type 1 Diabetes Mellitus
-
Protocol number: CT7001-002
AN OPEN-LABEL INTERVENTIONAL, MULTICENTER, RANDOMIZED, PHASE 2 STUDY OF FULVESTRANT WITH OR WITHOUT SAMURACICLIB IN PARTICIPANTS WITH METASTATIC OR LOCALLY ADVANCED HORMONE RECEPTOR POSITIVE AND HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2-NEGATIVE BREAST CANCER
-
Protocol number: CVAY736Q12201
A phase 2 study of ianalumab (VAY736) in patients with primary immune thrombocytopenia (ITP) previously treated with at least two lines of therapies
-
Protocol number: CVRX-BAROSTIMTHERAPY
BAROSTIM THERAPY In Heart Failure with Reduced Ejection Fraction A Post-Market Registry with the BAROSTIM NEO System
-
Protocol number: CYTOKINETICS-CY6022
An Open-Label Study of CK-3773274 for Patients with Symptomatic Hypertrophic Cardiomyopathy (HCM)
-
Protocol number: D4191C00140-NCT05771480
A Phase IIIb, Single Arm, Open-label, Multicentre Study of Durvalumab in Combination with Chemotherapy for the First Line Treatment for Patients with Advanced Biliary Tract Cancers
-
Protocol number: D7000C00001
SUPERNOVA (Study Understanding Pre-Exposure pRophylaxis of NOVel Antibodies) - AZD5156/AZD3152 - D7000C00001
-
Protocol number: D910PC00001-VOLGA
A Phase III Randomized, Open-Label, Multicenter Study to Determine the Efficacy and Safety of Durvalumab in Combination With Tremelimumab and Enfortumab Vedotin or Durvalumab in Combination With Enfortumab Vedotin for Perioperative Treatment in Patients Ineligible for Cisplatin Undergoing Radical Cystectomy for Muscle Invasive Bladder Cancer (VOLGA)
-
Protocol number: D926PC00001-TROPION-02
A Phase 3, Open-label, Randomised Study of Datopotamab Deruxtecan (Dato-DXd) VersusInvestigator s Choice of Chemotherapy in Patients who are not Candidates for PD-1/PD-L1 Inhibitor Therapy in First-line Locally Recurrent Inoperable or Metastatic Triple-negative Breast Cancer (TROPION-Breast02)
-
Protocol number: D926XC00001-NCT05629585
A Phase 3 Open-label, Randomised Study of Datopotamab Deruxtecan (DatoDXd) With or Without Durvalumab Versus Investigator's Choice of Therapy in Patients With Stage I-III Triple-negative Breast Cancer Who Have Residual Invasive Disease in the Breast and/or Axillary Lymph Nodes at Surgical Resection Following Neoadjuvant Systemic Therapy (TROPION-Breast03)
-
Protocol number: DARANIVOVAX-2022-NCT06015724
A Phase 2 Study Evaluating the Efficacy of Anti-CD38 antibody in Combination with KRAS vaccine and Anti-PD-1 Antibody in Subjects with Pancreatic Ductal Adenocarcinoma and Refractory Non-Small Cell Lung Cancer
-
Protocol number: DARATUMUMAB-MGUS-NCT06046287
Phase IIa Study to Evaluate Daratumumab for Polyneuropathy Associated with MGUS
-
Protocol number: DB-1303-O-1001-NCT05150691
A Phase 1/2a Multicenter, Open-Label, Non-Randomized First in Human Study to Assess the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of DB-1303 in Patients with Advanced/Metastatic Solid Tumors
-
Protocol number: DB-EF-PHASEIII-0001
Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles for Hospitalized Patients with Respiratory Failure due to Severe or Critical COVID-19, Including Those with Moderate-to-Severe ARDS: A Phase III Clinical Trial.
-
Protocol number: DCL-16-001-NCT02952508
An Open-Label, Multicenter, Phase 2 Study of CLR 131 in Patients with Relapsed or Refractory (R/R) Select B-Cell Malignancies (CLOVER-I) and Expansion Cohort in Patients with Waldenstrom Macroglobulinemia (CLOVER-WaM)
-
Protocol number: DCM-CMR-STUDY
Dilated Cardiomyopathy (DCM) Research Project Sub-study: Advanced Procedures Sub-Study
-
Protocol number: DEFINE-GPS
Distal Evaluation of Functional performance with Intravascular sensors to assess the Narrowing Effect: Guided Physiologic Stenting
-
Protocol number: DEXTERITY-CIP0217
DEXTERITY-AFP: Perivenous Dexamethasone Therapy: Examining Reduction of Inflammation after Thrombus Removal to Yield Benefit in Acute Femoropopliteal DVT
-
Protocol number: DEXTERITY-CIP0218
DEXTERITY-SCI: Perivenous Dexamethasone Therapy: Examining Reduction of Inflammation after Thrombus Removal to Yield Benefit in Subacute and Chronic Iliofemoral DVT
-
Protocol number: DF/HCC-19-202-NCT04025372
INTREPId (INTermediate Risk Erection PreservatIon Trial : A Randomized Phase II Trial of Radiation Therapy and Darolutamide for Prostate Cancer.
-
Protocol number: DIRECTION-NCT04936542
A Multicentre, Interventional, Post-marketing, Randomised, Double-blind, Crossover Study to Evaluate the Clinical Safety and Efficacy of AbobotulinumtoxinA (Dysport®) in Comparison with OnabotulinumtoxinA (Botox®) when Treating Adults with Upper Limb Spasticity
-
Protocol number: DISCOVER-HCM
Observational Study Protocol CV027-012: DELIVER INSIGHTS IN HYPERTROPHIC CARDIOMYOPATHY AND OBSERVATIONAL OUTCOMES IN REAL WORLD (DISCOVER-HCM): UNITED STATES PROSPECTIVE REGISTRY STUDY
-
Protocol number: DISRUPT-CAD-DUO
Prospective, Multicenter, Single-Arm IDE Study of the Shockwave Coronary Intravascular Lithotripsy (IVL) System with the Shockwave C2+ 2Hz Coronary IVL Catheter in Calcified Coronary Arteries (Disrupt CAD Duo Study)
-
Protocol number: DISRUPTPAD-BTKII
Prospective, Multi-center, Single-arm Study of the Shockwave Medical Peripheral Intravascular Lithotripsy (IVL) System for Treatment of Calcified Peripheral Arterial Disease (PAD) in Below-the-Knee (BTK) Arteries
-
Protocol number: DLS-NCTNA
Collection of Bio-fluid Samples from Pregnant Women for the Evaluation of Biomarkers for Preeclampsia
-
Protocol number: DOD-ESTIMATEHEALTHYASYMPTO
DOD- Estimate healthy, asymptomatic urine state variability of urine NGAL (uNGAL), white blood cells (uWBC), nitrite, cultivable bacteria, and the urinary microbiome-AIM 1
-
Protocol number: DOTS
Food and Drug Administration (FDA) Toxicology Investigator's Consortium (ToxIC) Drug Overdose Toxico-Surveillance (DOTS) Reporting Program.
-
Protocol number: DRESSINGCOMPARRISON
A Comparison of Split-thickness Skin Graft Primary Dressings When Used with Autologous Skin Cell Suspension.
-
Protocol number: DUET-CD
A Phase 2b Randomized, Double-blind, Active- and Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Induction and Maintenance Combination Therapy with Guselkumab and Golimumab in Participants with Moderately to Severely Active Crohn’s Disease”
-
Protocol number: DUET-UC
A Phase 2b Randomized, Double-blind, Active- and Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Induction and Maintenance Combination Therapy with Guselkumab and Golimumab in Participants with Moderately to Severely Active Ulcerative Colitis.
-
Protocol number: DURECTAH
A Randomized, Double-blind, Placebo-controlled, Phase 2b study to Evaluate Safety and Efficacy of DUR-928 in Subjects with Alcoholic Hepatitis
-
Protocol number: EA1151-TMIST-NCT03233191
Tomosynthesis Mammographic Imaging Screening Trial (TMIST) EA 1151
-
Protocol number: EA2182-NCT04166318
A Randomized Phase II Study of De-Intensified ChemoRadiation for Early-Stage Anal Squamous Cell Carcinoma (DECREASE)
-
Protocol number: EA2185-PANCREATIC-NCT04239573
Comparing Two Methods to Follow Patients With Pancreatic Cysts
-
Protocol number: EA3191
A Phase II Randomized Trial of Adjuvant Therapy With Pembrolizumab After Resection of Recurrent/Second Primary Head and Neck Squamous Cell Carcinoma With High Risk Features
-
Protocol number: EA3202
A Phase II/III Trial of Chemotherapy + Cetuximab vs Chemotherapy + Bevacizumab vs Atezolizumab + Bevacizumab Following Progression on Immune Checkpoint Inhibition in Recurrent/Metastatic Head and Neck Cancers
-
Protocol number: EA6141-NCT02339571
Randomized Phase II/III Study of Nivolumab Plus Ipilimumab Plus Sargramostim Versus Nivolumab Plus Ipilimumab in Patients With Unresectable Stage III or Stage IV Melanoma
-
Protocol number: EA6192
A Phase II Study of Biomarker Driven Early Discontinuation of Anti-PD-1 Therapy in Patients with Advanced Melanoma (PET-Stop)
-
Protocol number: EA8184
A Phase II Randomized Double Blinded Study of Green Tea Catechins (GTC) vs. Placebo in Men on Active Surveillance for Prostate Cancer: Modulation of Biological and Clinical Intermediate Biomarkers
-
Protocol number: EA8192
A Phase II/III Trial of MEDI4736 (Durvalumab) and Chemotherapy for Patients With High Grade Upper Tract Urothelial Cancer Prior to Nephroureterectomy
-
Protocol number: EA8212-BRIDGE
EA8212: A Randomized Phase III Trial of Intravesical BCG veRsus Intravesical Docetaxel and GEmcitabine Treatment in BCG Naïve High Grade Non-Muscle Invasive Bladder Cancer (BRIDGE)
-
Protocol number: EASESBS2EXT-NCT03905707
A Double-Blind Phase 3 Extension Trial Assessing the Long-Term Safety and Efficacy of Glepaglutide in Patients with Short Bowel
-
Protocol number: EASESBS3EXT-NCT04991311
A 104-week, multicenter, single-arm, long-term, phase 3 extension trial investigating the safety and efficacy of Glepaglutide in adult patients with short bowel syndrome (SBS) completing the EASE SBS 2 trial
-
Protocol number: EAST-002
Randomized Controlled Clinical Trial (RCT) of the Nectero EAST System for Small to Mid-Sized Abdominal Aortic Aneurysms (AAA) StaBiLization: Evaluation of Efficacy.
-
Protocol number: EBO-301
A Phase 2/3, Randomized, Double-blind, Placebo-controlled, Multicenter, Prospective Study to Assess the Efficacy, Safety, and Pharmacokinetics of Orally Administered Epetraborole in Patients with Treatment-refractory Mycobacterium avium Complex Lung Disease (MACrO2)
-
Protocol number: EBONI
ViiV Healthcare / “A phase 4, randomized, open-label, three arm study to evaluate implementation strategies for the delivery of CAB for HIV Pre-exposure Prophylaxis (PrEP) across clinical settings for adult (≥18 years) Black cis-and transgender women without HIV infection living in the United States Ending the Epidemic (EHE) territories”
-
Protocol number: ECUNMO303-NCT04155424
A Phase 2/3 Open-Label, Single-Arm Trial to Evaluate the Safety and Activity of Eculizumab in Pediatric Patients with Relapsing Neuromyelitis Optica Spectrum Disorder
-
Protocol number: EIK1001-005-FSQ
A Phase 2 Study of EIK1001 in Combination with Pembrolizumab and Chemotherapy in Patients with Stage 4 Non-Small Cell Lung Cancer
-
Protocol number: EIP21-NFD-504
A Phase 2b Clinical Study of the P38 Alpha Kinase Inhibitor Neflamapimod in Patients with Dementia with Lewy Bodies (DLB)
-
Protocol number: EKOBLANKETPROTOCOL
Evaluation of Eko hardware, software, and algorithm performance across multiple scenarios, use cases, and device generations
-
Protocol number: ELA026-CP002
A Phase 1b, Open-label, Single-arm, Multicenter Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of Multiple Doses of ELA026 in Adults and Adolescents with Secondary Hemophagocytic Lymphohistiocytosis (sHLH)
-
Protocol number: ELEGANCE-REGISTRY
ELEGANCE Drug-ELuting REGistry: ReAl-World Treatment of LesioNs in the Peripheral VasCulaturE
-
Protocol number: EMBLOK
A Prospective, Randomized, Multicenter Evaluation of the Safety and Effectiveness of the EMBLOK™ Embolic Protection System during Transcatheter Aortic Valve Replacement
-
Protocol number: ENCIRCLE-NCT04153292
The ENCIRCLE Trial SAPIEN M3 System TransCatheter MItral Valve ReplaCement via TransseptaL AccEss
-
Protocol number: ENLIGHTEN-NCT04140305
A Multicenter, Longitudinal, Open-Label, Single-Arm Study Describing Cognitive Processing Speed Changes in Relapsing Multiple Sclerosis Subjects Treated With Ozanimod (RPC-1063)
-
Protocol number: EP0031-101
A Modular, Open-label, Phase I/II Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of EP0031 in Patients With Advanced RET-altered Malignancies
-
Protocol number: EPITOME-CLIN-52120-458-NCT0605
A PROSPECTIVE, MULTICOUNTRY STUDY TO ESTIMATE THE INCIDENCE OF AND PROVIDE A BEST PRACTICE MODEL FOR MONITORING THE DEVELOPMENT OF POST-STROKE SPASTICITY
-
Protocol number: EQU-202
A Randomized Phase 2 Study of Adjunctive EQU-001 for Uncontrolled Focal Onset Seizures
-
Protocol number: ES201601-NCTNA
Blood Sample Collection in Subjects with Pulmonary Nodules or CT Suspicion of Lung Cancer
-
Protocol number: ESR-18-13870
A Phase II Trial of Durvalumab (MEDI4736)and Tremelimumab in Combination With Chemotherapy in Virus-infected Patients With Non-small Cell Lung Cancer
-
Protocol number: ESR-22-21719-NCT06017297
Phase II Study of Neoadjuvant Durvalumab (MEDI4736) and Tremelimumab in Combination with Gemcitabine and Cisplatin in Patients with Borderline Resectable/Locally Advanced Cholangiocarcinoma.
-
Protocol number: ETCTN-10204-NCT03816345
A Phase Ib Study of Nivolumab in Patients with Autoimmune Disorders and Advanced Malignancies (AIM-NIVO).
-
Protocol number: ETCTN-10276-NCT04068194
A Phase I/II Study of M3814 and Avelumab in Combination with Hypofractionated Radiation in Patients with Advanced/Metastatic Solid Tumors and Hepatobiliary Malignancies
-
Protocol number: EV-ICD-CAS
ExtraVascular Implantable Cardiac Defibrillator (EV ICD) Continued Access Study
-
Protocol number: EXA2202-NCT05796999
ExaStim Upper Limb Pivotal Clinical Validation Study
-
Protocol number: EXT00000206-PRELUDE
A Prospective Registry Study to Evaluate the Effect of the DCISionRT Test on Treatment Decisions in Patients with DCIS Following Breast Conserving Therapy
-
Protocol number: EXTEND-001
Thoraflex Hybrid and Relay Extension Post-Approval Study (EXTEND)
-
Protocol number: FARAPULSE-ADVENT-NCT04612244
A Prospective Randomized Pivotal Trial of the FARAPULSE Pulsed Field Ablation System Compared with Standard of Care Ablation in Patients with Paroxysmal Atrial Fibrillation
-
Protocol number: FAST
Functional Arm Use After Stroke (FAST) as a Measure of Motor Recovery After Stroke
-
Protocol number: FAST-ULTRASOUND
Abdominal FAST imaging of Adult Trauma Patients to Build an Annotated Ultrasound Image Library for Development of Automated Ultrasound Image Acquisition and Interpretation Algorithms.
-
Protocol number: FASTR-RCV-0006
Fluid management of Acute decompensated heart failure Subjects Treated with Reprieve Decongestion Management System (DMS)
-
Protocol number: FENTREPID
A PHASE III MULTICENTER, RANDOMIZED, DOUBLE-BLIND, DOUBLE-DUMMY, PARALLEL-GROUP STUDY TO EVALUATE THE EFFICACY AND SAFETY OF FENEBRUTINIB COMPARED WITH OCRELIZUMAB IN ADULT PATIENTS WITH PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS
-
Protocol number: FEVAR-NCT04526938
Clinical Outcomes, Radiation Dosage, and Quality of Life in Patients Treated with Fenestrated Stent Grafts for Complex Thoracoabdominal and Abdominal Aortic Aneurysms, and Thoracoabdominal Aortic Aneurysms secondary to Aortic Dissections
-
Protocol number: FORWARD-IDE
Forward-Shifted Intravascular Lithotripsy (IVL) Technology in a Prospective, Multi-center, Single-arm Investigational Device Exemption (IDE) Study (FORWARD IDE Study)
-
Protocol number: G-LEAGUE
Understanding the Impact of a G League Season on Player Health, Performance and Patellar Tendinopathy: A Pilot Observational Study
-
Protocol number: GA43360
A Phase 1b, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Multiple-Ascending Doses of RO7303509 in Patients with Systemic Sclerosis
-
Protocol number: GEMINI-1-NCT04410978
A Phase 3, randomized, double-blind efficacy and safety study comparing SAR442168 to teriflunomide (Aubagio®) in participants with relapsing forms of multiple sclerosis (GEMINI 1)
-
Protocol number: GENENTECHML43702
LONG-TERM FOLLOW-UP STUDY OF PATIENTS WITH SPINAL MUSCULAR ATROPHY RECEIVING RISDIPLAM TREATMENT
-
Protocol number: GENERATION1-NCT02565511
A Randomized, Double-blind, Placebo-controlled, Two-cohort, Parallel Group Study to Evaluate the Efficacy of CAD106 and CNP520 in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer's Disease.
-
Protocol number: GN42272
A PHASE III MULTICENTER, RANDOMIZED, DOUBLE-BLIND, DOUBLE-DUMMY, PARALLEL-GROUP STUDY TO EVALUATE THE EFFICACY AND SAFETY OF FENEBRUTINIB COMPARED WITH TERIFLUNOMIDE IN ADULT PATIENTS WITH RELAPSING MULTIPLE SCLEROSIS
-
Protocol number: GO42216
A PHASE Ib/II, OPEN-LABEL, MULTICENTER, RANDOMIZED UMBRELLA STUDY EVALUATING THE EFFICACY AND SAFETY OF MULTIPLE IMMUNOTHERAPY-BASED TREATMENT COMBINATIONS IN PATIENTS WITH ADVANCED LIVER CANCERS (MORPHEUS-LIVER)
-
Protocol number: GO44457-NCT04524871
A PHASE Ib/II, OPEN-LABEL, MULTICENTER, RANDOMIZED PLATFORM STUDY EVALUATING THE EFFICACY AND SAFETY OF NEOADJUVANT IMMUNOTHERAPY COMBINATIONS IN PATIENTS WITH SURGICALLY RESECTABLE HEPATOCEULLULAR CARCINOMA
-
Protocol number: GORE-VIAFORT-NCT05409976
Evaluation of the GORE® VIAFORT Vascular Stent for Treatment of Symptomatic Inferior Vena Cava Obstruction with or without Combined Iliofemoral Obstruction
-
Protocol number: GORETAG-NCT02777528
Evaluation of the GORE® TAG® Thoracic Branch Endoprosthesis (TBE Device) in the Treatment of Lesions of the Aortic Arch and Descending Thoracic Aorta
-
Protocol number: GORETAMBE-NCT03728985
Evaluation of the GORE® EXCLUDER® Thoracoabdominal Branch Endoprosthesis in the Treatment of Thoracoabdominal and Pararenal Aortic Aneurysms
-
Protocol number: GP-88
Serum GP-88 Levels in Metastatic Breast Cancer patients: A Prospective Blood Sampling Trial
-
Protocol number: GRAIL-02-PATHFINDER-2
The PATHFINDER 2 Study: Evaluating the Safety and Performance of the GRAIL Multi-Cancer Early Detection Test in an Eligible Screening Population
-
Protocol number: GS-US-200-4334
A Phase 2 Randomized, Open Label, Active Controlled Study Evaluating the Safety and Efficacy of Long-acting Capsid Inhibitor GS-6207 in Combination with Other Antiretroviral Agents in People Living with HIV
-
Protocol number: GS-US-425-6143
GS-US-425-6143 - A Phase 1b Randomized, Blinded, Placebo-Controlled, Single and Multiple Ascending Dose Study to Evaluate the Safety, Tolerability and Pharmacokinetics of GS-8588 in People with HIV-1 who are Virologically Suppressed on Antiretroviral Therapy
-
Protocol number: GS-US-536-5939
A Phase 2 Study of GS-5423 and GS-2872 in Combination With Capsid Inhibitor Lenacapavir in Virologically Suppressed Adults With HIV-1 Infection
-
Protocol number: GS-US-595-6184-ASCENT-05
A Randomized, Open-label, Phase 3 Study of Adjuvant Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician s Choice in Patients With Triple Negative Breast Cancer Who Have Residual Invasive Disease After Surgery and Neoadjuvant Therapy
-
Protocol number: GS-US-611-6549
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of GS-5245 for the Treatment of COVID-19
-
Protocol number: GSK-219369-AZUR-1
A Phase 2, Single-Arm, Open-Label Study with Dostarlimab Monotherapy in Participants with Untreated Stage II/III dMMR/MSI-H Locally Advanced Rectal Cancer
-
Protocol number: GSK-219606-AZUR-2
A Phase 3, Open-Label, Randomized Study of Perioperative Dostarlimab Monotherapy versus Standard of Care in Participants with Untreated T4N0 or Stage III dMMR/MSI-H Resectable Colon Cancer
-
Protocol number: GSK-221530-SCC
A Randomized, Double-blind, Placebo-controlled Phase 3 Study to evaluate Dostarlimab as Sequential Therapy after Chemoradiation in Participants with Locally Advanced Unresected Head and Neck Squamous Cell Carcinoma
-
Protocol number: GU16-260-NCT03117309
Phase II Study of Front Line Therapy With Nivolumab and Salvage Nivolumab + Ipilimumab in Patients With Advanced Renal Cell Carcinoma. HCRN: GU16-260
-
Protocol number: GUH-CDF-STUDY
Feasibility Study to Understand Screening and Treatment Rates for Cognitive Impairment, Depression, and Fatigue in a Large, Urban MS Comprehensive Care Center
-
Protocol number: HA-PEEK
A Multi-center, Patient Outcome Registry for a Hydroxyapatite infused PEEK Interbody Fusion Device
-
Protocol number: HBVFB
Impact of Recent Immigration on Delays in Care Delivery among Foreign-Born with Chronic Hepatitis B Infection
-
Protocol number: HCRN-GU22-587-NCT05928806
Advanced Renal Cell Cancer Combination ImmunoThErapy Clinical Trial (ARCITECT)
-
Protocol number: HEAL-IST
Hybrid Epicardial and Endocardial Sinus-Node SpAring AbLation Therapy for Inappropriate Sinus Tachycardia
-
Protocol number: HEAL-LAA
Post-Market Real World Outcomes in WATCHMAN FLX Pro Left Atrial Appendage Closure (LAAC) Device
-
Protocol number: HEM-PWRV1US
HEM-POWR: Observational Study Evaluating Effectiveness and Safety of Real-World Treatment with Damoctocog alfa pegol in Previously Treated Patients with Hemophilia A
-
Protocol number: HEP3
Human Epilepsy Project 3: Newly Diagnosed Idiopathic Generalized Epilepsy
-
Protocol number: HIALIVER-NCT03734393
HOPE in Action Prospective Multicenter, Clinical Trial of HIV+ Deceased Donor Liver Transplants for HIV+ Recipients
-
Protocol number: HP-00081403-NCT03652428
Phase I Study of Concurrent Nab-Paclitaxel + Gemcitabine With Hypofractionated, Ablative Proton Therapy for Locally Advanced Pancreatic Cancer
-
Protocol number: HSG001
Development of the Virtual Unified Huntington Disease Rating Scale (vUHDRS)
-
Protocol number: I-SPY2-NCT01042379
I-SPY Trial (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging And moLecular Analysis 2)
-
Protocol number: I5T-MC-AACI
Assessment of Safety, Tolerability, and Efficacy of Donanemab in Early Symptomatic Alzheimer's Disease
-
Protocol number: ICG-SCAPHOID
Use of Indocyanine Green intraoperatively to examine vascularity of scaphoid with histological comparison
-
Protocol number: IDEAS-NCT02420756
Imaging DementiaEvidence for Amyloid Scanning (IDEAS) Study: A Coverage With Evidence Development Longitudinal Cohort Study
-
Protocol number: IMC-F106C-101-NCT04262466
A Phase 1/2 First-in-Human Study of the Safety and Efficacy of IMC-F106C as a Single Agent and in Combination with Checkpoint Inhibitors in HLA-A*02:01-Positive Participants with Advanced PRAME-Positive Cancers
-
Protocol number: IMMUKNOW
Utility of ImmuKnow, an Immune Cell Function Assay, in a Cardiac Sarcoid Population
-
Protocol number: IMPROVE-NCT04221815
IMPact on Revascularization Outcomes of intraVascular ultrasound guided treatment of complex lesions and Economic impact (IMPROVE)
-
Protocol number: IMPROVEMENT-NCT705289609
International, Multicenter, Prospective, Non-competitive, Observational study to Validate and Optimize prediction models of 90-day and 1-year allograft failure after liver transplantation
-
Protocol number: IMVT-1401-2401
A Phase 2b, Multi-center, Randomized, Quadruple-blind, Placebo-controlled Study of Batoclimab Treatment in Adult Participants with Active Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
-
Protocol number: INCB18424-369
A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Assess the Efficacy and Safety of Ruxolitinib in Participants With COVID-19Associated ARDS Who Require Mechanical Ventilation (RUXCOVID-DEVENT)
-
Protocol number: INF-04-NCT04094818
HostDx Sepsis in the Diagnosis and Prognosis of Emergency Department Patients With Suspected Infections and Suspected Sepsis (SEPSIS-SHIELD)
-
Protocol number: INS-416
ENCORE - A Randomized, Double-Blind, Placebo-Controlled, Active Comparator, Multicenter Study to Evaluate the Efficacy and Safety of an Amikacin Liposome Inhalation Suspension (ALIS)-Based Regimen in Adult Subjects with Newly Diagnosed Nontuberculous Mycobacterial (NTM) Lung Infection Caused by Mycobacterium avium Complex (MAC)
-
Protocol number: INSIGHT018-A
A Multicenter, Adaptive, Randomized, Controlled Trial Platform To Evaluate Safety and Efficacy of Strategies and Treatments for Hospitalized Patients with Respiratory Infections
-
Protocol number: INSMED312-NCT02628600
An Open-Label Safety Extension Study to a Multicenter Study of Liposomal Amikacin for Inhalation (LAI) in Adult Patients with Nontuberculous Mycobacterial (NTM) Lung Infections caused by Mycobacterium avium complex (MAC) that are refractory to treatment
-
Protocol number: INTEGRA2017-0908
The Relationship Between Bacterial Load and the Clinical Outcomes for Integra in Operative Wounds
-
Protocol number: INTRAVESICALLGGVERSUSSALINE
Intravesical Lactobacillus rhamnosusGG versus saline bladder wash: A randomized, controlled, comparative effectiveness clinical trial
-
Protocol number: ISIS702843CS4
A Phase 2a, Randomized, Open-Label Study to Evaluate the Efficacy, Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of ISIS 702843 Administered to Patients with Phlebotomy Dependent Polycythemia Vera (PD-PV)
-
Protocol number: ISPYCOVID
I-SPY COVID TRIAL: An Adaptive Platform Trial to Reduce Mortality and Ventilator Requirements for Critically Ill Patients
-
Protocol number: ISS-20207331-NCT05199311
A Phase I/II Study of Carfilzomib, Iberdomide (CC-220) and Dexamethasone (KID) in Patients With Newly Diagnosed Transplant Eligible Multiple Myeloma
-
Protocol number: ISS-59074-PEMBRO-LENV
A phase II trial of combination therapy of pembrolizumab and Lenvatinib in patients with locally advanced or metastatic cervical cancer
-
Protocol number: ISTRADEFYLLINECHARTREVIEW
Effectiveness of Istradefylline on Motor Functions in Patients with Parkinson's Disease
-
Protocol number: J1I-MC-GZBZ
A Phase 3, Randomized, Multicenter, Open-label Study to Investigate the Efficacy and Safety of Retatrutide Once Weekly Compared with Semaglutide Once Weekly in Adult Participants with Type 2 Diabetes and Inadequate Glycemic Control with Metformin with or without SGLT2 Inhibitor
-
Protocol number: J2A-MC-GZGP
A Phase 3, Randomized, Double-Blind Study to Investigate the Efficacy and Safety of Once-Daily Oral LY3502970 Compared with Placebo in Adult Participants with Obesity or Overweight with Weight-Related Comorbidities
-
Protocol number: J2A-MC-GZGQ
A Phase 3, Randomized, Double-Blind Study to Investigate the Efficacy and Safety of Once-Daily Oral LY3502970 Compared with Placebo in Adult Participants with Obesity or Overweight and Type 2 Diabetes (ATTAIN-2)
-
Protocol number: J2J-MC-JZLH-EMBER-4
EMBER-4: A Randomized, Open-Label, Phase 3 Study of Adjuvant Imlunestrant vs Standard Adjuvant Endocrine Therapy in Patients who have Previously Received 2 to 5 years of Adjuvant Endocrine Therapy for ER+, HER2- Early Breast Cancer with an Increased Risk of Recurrence
-
Protocol number: J2W-MC-PYAB
A Randomized, Double-blind, Placebo-Controlled, Phase 2 Study to Evaluate the Efficacy and Safety of LY3819253 in Participants with Mild to Moderate COVID-19 Illness
-
Protocol number: J2X-MC-PYAH
A Randomized, Double-blind, Placebo-Controlled, Phase 2 Study to Evaluate the Efficacy and Safety of Mono and Combination Therapy with Monoclonal Antibodies in Participants with Mild to Moderate COVID-19 Illness (BLAZE-4)
-
Protocol number: JAGUAR-CP-0017
ObJective Analysis to GaUge EVAR Outcomes Through Randomization
-
Protocol number: JANSSEN-70033093STR3001
A Phase 3, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, for Stroke Prevention after an Acute Ischemic Stroke or High-Risk Transient Ischemic Attack
-
Protocol number: JENAVALVE-AR-NCT02732704
JenaValve Pericardial TAVR Aortic Regurgitation Study
-
Protocol number: JENAVALVE-AS-NCT02732691
JenaValve Pericardial TAVR System Aortic Stenosis Study
-
Protocol number: JIDDU
Validating the Jiddu Analyzer for Urine Bacterial Growth in Neurogenic Lower Urinary Tract Dysfunction
-
Protocol number: JNJ-70033093
A Phase 3, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, for Stroke Prevention after an Acute Ischemic Stroke or High-Risk Transient Ischemic Attack
-
Protocol number: JUGGERKNOT-PMS
JuggerKnot with BroadBand Tape Post Market Clinical Follow-up (PMCF) Study
-
Protocol number: JUVEENA
Safety and Effectiveness of Juveena Hydrogel System Following Transcervical Gynecologic Procedures (TCGP) At High-Risk for Intrauterine Adhesions: A Multicenter Randomized Controlled Subject and Evaluator Blinded Pivotal Study.
-
Protocol number: KEYNOTE-992-NCT04241185
A Phase 3, Randomized, Double-blind, Placebo-controlled Clinical Trial to Study the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination With Chemoradiotherapy (CRT) Versus CRT Alone in Participants With Muscle-invasive Bladder Cancer (MIBC) (KEYNOTE-992)
-
Protocol number: KIDNEY-OCT-NCT00000000
Automatic Wide-Field Optical Coherence Tomography for Assessment of Transplant Kidney Viability
-
Protocol number: KUPFER-BM-3680
Collection of blood and bone marrow from patients with genetic blood and bone marrow disease
-
Protocol number: LADD
Evaluation of Laser-assisted Drug Delivery for the Treatment of Hypertrophic Scar Hypopigmentation: A Within Patient-Controlled Trial in Skin of Color
-
Protocol number: LEAAPS-CP-2021-05
Left Atrial Appendage Exclusion for Prophylactic Stroke Reduction Trial
-
Protocol number: LEARN-NCT02488720
Longitudinal Evaluation of Amyloid Risk and Neurodegeneration - the LEARN Study. A Companion Observational Study to Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) Trial
-
Protocol number: LIMB-ISCHEMIA
Prospective Study Evaluating the Effect of Skin Pigmentation on Clinical Exam for Determination of Limb Ischemia in Patients with Digital Injuries
-
Protocol number: LITERACY
Limited Health Literacy in Patients with Rotator Cuff Tears: Risk Factors and Outcome Effects
-
Protocol number: LPX-641-001
A multicenter observational study for Immune-phenotyping and ex-vivo evaluation of the likelihood of response to an experimental tolerance restoration therapy in subjects diagnosed with multiple sclerosis (MS).
-
Protocol number: LYL797-101-NCT05274451
A Phase 1 Study to Assess the Safety and Efficacy of LYL797, ROR1-Targeting CAR T Cells, in Adults with Relapsed and/or Refractory Solid-Tumor Malignancies
-
Protocol number: LYL845-101-NCT05573035
A Phase 1 Study to Assess the Safety and Efficacy of LYL845 in Adults with Relapsed and/or Refractory Metastatic or Locally Advanced Melanoma and Selected Solid Tumor Malignancies
-
Protocol number: M19-148
A Randomized, Double-Blind, Placebo-Controlled Proof-of-Concept Study to Assess the Safety and Efficacy of Elezanumab in Acute Ischemic Stroke
-
Protocol number: M21-307/NCT05028569
Episodic Migraine: Phase 3 Study of BOTOX (Botulinum Toxin Type A) for the Prevention of Migraine in Subjects with Episodic Migraine
-
Protocol number: M21-459
A Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel Arm Study to Assess the Safety and Efficacy of a Single Treatment of BOTOX®, Followed by an Optional Open-Label Treatment with BOTOX®, in Female Subjects with Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS)
-
Protocol number: M23-499-NCT05956509
A Randomized, Double-blind, Placebo-controlled Study to Evaluate Safety, Efficacy, and Tolerability of ABBV-950 for the Treatment of Upper Limb Spasticity in Adult Post-Stroke Patients
-
Protocol number: M602011069
Prospective, randomized, double-blind, placebo-controlled, parallel-group trial with an open-label period to investigate the efficacy and safety of NT 201 in the unilateral and bilateral treatment of essential tremor of the upper limb
-
Protocol number: MAPLE-NCT04793360
Molecular Assessment and Profiling of Liver Transplant Recipients
-
Protocol number: MARCH
Randomized Double-blind Placebo-controlled Phase 3 Study to Evaluate the Efficacy and Safety of Maralixibat in the Treatment of Subjects with Progressive Familial Intrahepatic Cholestasis
-
Protocol number: MATCH-STUDY
Measurements of AV Access Blood Flow with the Alio Medical Remote Monitoring System to Determine Maturation Status and Patency in ESRD Patients
-
Protocol number: MCLA-128-CL01-NCT02912949
A Phase I/II Study of MCLA-128, a full length IgG1 Bispecific Antibody Targeting HER2 and HER3, in Patients with Solid Tumors
-
Protocol number: MGC018-ES-SCLC-NCT06227546
A PHASE II STUDY OF MGC018 IN PATIENTS WITH RELAPSED OR REFRACTORY EXTENSIVE-STAGE SMALL-CELL LUNG CANCER (ES-SCLC)
-
Protocol number: MITRAL2-NCT04408430
The safety and effectiveness of the SAPIEN 3 and SAPIEN 3 Ultra valve with Commander delivery system in patients with symptomatic severe calcific mitral valve disease with severe mitral annular calcification who are not candidates for standard mitral valve surgery.
-
Protocol number: MK-2870-005-GOG-3095
A Phase 3, Randomized, Active-controlled, Open-label, Multicenter Study to Compare the Efficacy and Safety of MK-2870 Monotherapy Versus Treatment of Physician s Choice in Participants With Endometrial Cancer Who Have Received Prior Platinum-based Chemotherapy and Immunotherapy (MK-2870-005/ENGOT-en23/GOG 3095)
-
Protocol number: MK-3475-587
A Multicenter, Open label, Phase III Extension Trial to Study the Long-term Safety and Efficacy in Participants with Advanced Tumors Who Are Currently on Treatment or in Follow-up in a Pembrolizumab Trial.
-
Protocol number: MK-6482-024
A multicenter, open-label, Randomized Phase 1/2 Study of belzutifan in combination with palbociclib vs belzutifan monotherapy in participants with advanced RCC
-
Protocol number: MK-7684A-010-01-NCT05665595
A Phase 3, Randomized, Double-blind, Active-Comparator-Controlled Clinical Study of Adjuvant MK-7684A (Vibostolimab with Pembrolizumab) Versus Adjuvant Pembrolizumab in Participants with High-risk Stage II-IV Melanoma (KEYVIBE-010)
-
Protocol number: MK-7902-009-05-NCT04428151
A Phase 2, randomized, open-label three-arm clinical study to evaluate the safety and efficacy of lenvatinib (E7080/MK-7902) in combination with pembrolizumab (MK-3475) versus standard of care chemotherapy and lenvatinib monotherapy in participants with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) that have progressed after platinum therapy and immunotherapy (PD-1/PD-L1 inhibitors)
-
Protocol number: MK-7902-014-03
A Phase 3, Randomized Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK-3475) + Lenvatinib (E7080/MK-7902) + Chemotherapy Compared with Standard of Care as First-line Intervention in Participants with Metastatic Esophageal Carcinoma
-
Protocol number: MK-8591A-017
A Phase 3, Randomized, Active-Controlled, Open-label Clinical Study to Evaluate a Switch to Doravirine/Islatravir (DOR/ISL) Once-Daily in Participants With HIV-1 Virologically Suppressed on Antiretroviral Therapy
-
Protocol number: MK-8591A-019
A Phase 3, Randomized, Clinical Study in HIV-1-Infected Heavily Treatment-Experienced Participants Evaluating the Antiretroviral Activity of Blinded Islatravir (ISL), Doravirine (DOR), and Doravirine/Islatravir (DOR/ISL), Each Compared to Placebo, and the Antiretroviral Activity, Safety, and Tolerability of Open-Label DOR/ISL
-
Protocol number: MK-8591A-051-00
A Phase 3, Randomized, Active-Controlled, Open-Label Clinical Study to Evaluate a Switch to Doravirine/Islatravir (DOR/ISL 100 mg/0.25 mg) Once-Daily in Participants With HIV-1 Who Are Virologically Suppressed on Antiretroviral Therapy
-
Protocol number: MK-8591A-054
A Phase 3 Open-label Clinical Study of Doravirine/Islatravir (DOR/ISL [100 mg/0.25 mg]) Once Daily for the Treatment of HIV-1
-
Protocol number: MK3475-365-NCT02861573
Phase Ib/II Trial of Pembrolizumab (MK-3475) Combination Therapies in Metastatic Castration-Resistant Prostate Cancer (mCRPC) (KEYNOTE-365)
-
Protocol number: MK3475-587
A Multicenter, Open label, Phase III Extension Trial to Study the Long-term Safety and Efficacy in Participants with Advanced Tumors Who Are Currently on Treatment or in Follow-up in a Pembrolizumab Trial
-
Protocol number: MK7339-013
A Randomized, Double-blind, Placebo-controlled Phase 3 Study of Pembrolizumab (MK-3475) in Combination With Concurrent Chemoradiation Therapy Followed by Pembrolizumab With or Without Olaparib (MK-7339), Compared to Concurrent Chemoradiation Therapy Alone in Participants With Newly Diagnosed Treatment-Naïve Limited-Stage Small Cell Lung Cancer (LS-SCLC)
-
Protocol number: MK7684A-006
Open-label Phase 3 Study of MK-7684A (Coformulation of Vibostolimab with Pembrolizumab) in Combination with Concurrent Chemoradiation followed by MK- 7684A Versus Concurrent Chemoradiation followed by Durvalumab in Participants with Unresectable, Locally-advanced, Stage III NSCLC Lung
-
Protocol number: ML42919
A Phase II Open-label Multi-cohort Study Evaluating the Efficacy of Tiragolumab with Atezolizumab plus Bevacizumab in Previously-Treated Advanced Non- squamous NSCLC
-
Protocol number: MOVR-NCT99999999
Muscular Dystrophy Association Neuromuscular Observational Research (MOVR) Data Hub Protocol Protocol# MDACS1
-
Protocol number: MRNA-4157-P201-NCT03897881
A Phase 2 Randomized Study of Adjuvant Immunotherapy With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab Versus Pembrolizumab Alone After Complete Resection of High-Risk Melanoma (KEYNOTE- 942)
-
Protocol number: MS-LINK-NEXTGEN
Next Generation Learning Health System for Multiple Sclerosis (Next-Gen MS): A prospective, cluster-randomized study evaluating the impact of feed-forward patient reported outcomes data to clinical teams managing adults living with MS in a learning health system for MS research.
-
Protocol number: MS-LINK-OUTCOMES
The MS Leadership and Innovation Network (MS-LINK ) Outcomes Study: A Comprehensive Prospective Longitudinal Assessment of Patient and Clinical Reported Outcomes in Multiple Sclerosis Patients across North America
-
Protocol number: MSK22-100-NCT05389293
An Open-Label, Multicenter, Single-Arm, Phase-2 Study of Single-Agent Mosunetuzumab (BTCT4465A, RO7030816) for the Treatment of Patients with Newly Diagnosed Follicular Lymphoma in Need of Systemic Therapy
-
Protocol number: MT-3921-A-001
A prospective multicenter, longitudinal, observational cohort study to assess the measurement properties of clinical outcomes assessments in patients with traumatic low cervical spinal cord injury
-
Protocol number: NAILNASH
Post-transplant NASH protocol to assess the development of metabolic co-morbidities and disease recurrence in those transplanted for NASH cirrhosis
-
Protocol number: NAPOLI-2-NCT04005339
Phase II Study of Fluorouracil, Leucovorin, and Nanoliposomal Irinotecan in Previously Treated Advanced Biliary Tract Cancer (NAPOLI-2)
-
Protocol number: NATURALHISTORY-NCT00009243
Evaluation, Pathogenesis and Treatment of Patients with or at Risk for Cerebrovascular Disease (A Natural History/Disease Pathogenesis Protocol)
-
Protocol number: NBI-98854-HD3006
Open-Label Rollover Study for Continuing Valbenazine Administration for the Treatment of Chorea Associated with Huntington Disease
-
Protocol number: NCT01892345
A Randomized, Double-Blind, Placebo-Controlled, Multi-Center Trial To Evaluate The Safety And Efficacy Of Eculizumab In Patients With Relapsing Neuromyelitis Optica (NMO)
-
Protocol number: NCT02242487
A 12-Month, Dose-Level Blinded Study Investigating the Safety and Efficacy of CVT-301 (Levodopa Inhalation Powder) in Parkinsons Disease Patients With Motor Response Fluctuations (OFF Phenomena)
-
Protocol number: NCT02901717
MDA 2013-0039:A Phase 3, Multi-Center, Randomized, Double-Blind Study to Evaluate the Efficacy and Safety of Mino-Lok Therapy (MLT) in Combination with Systemic Antibiotics in the Treatment of Catheter-Related or Central Line-Associated Bloodstream Infect
-
Protocol number: NEOD001-301-NCT04973137
A Phase 3, Randomized, Multicenter, Double-Blind, Placebo-Controlled, Efficacy and Safety Study of Birtamimab Plus Standard of Care vs. Placebo Plus Standard of Care in Mayo Stage IV Subjects with Light Chain (AL) Amyloidosis
-
Protocol number: NEROFE
Phase I Study of NEROFE and Doxorubicin in KRAS-mutated ST2-positive Solid Tumors
-
Protocol number: NEUROCOGNITION
Rate of Neurocognitive Decline in Adults with Sickle Cell Disease: an institutional Study
-
Protocol number: NGM707-IO-101
A Phase 1/2 Dose Escalation/Expansion Study of NGM707 as Monotherapy and in Combination With Pembrolizumab in Advanced or Metastatic Solid Tumor Malignancies
-
Protocol number: NIC-NCT02720445
Long Term Nicotine Treatment of Mild Cognitive Impairment
-
Protocol number: NOFEAR-BE-NCT03554356
Safety and Efficacy of the CryoBalloon Ablation for Treatment of Patients with Resistant Barrett's Esophagus (BE) - The Resistant BE Trial (ReBET)
-
Protocol number: NRG-BN009
PHASE III TRIAL OF SALVAGE STEREOTACTIC RADIOSURGERY (SRS) OR SRS + HIPPOCAMPALAVOIDANT WHOLE BRAIN RADIOTHERAPY (HA-WBRT) FOR FIRST OR SECOND DISTANT BRAIN RELAPSE AFTER UPFRONT SRS WITH BRAIN METASTASIS VELOCITY ≥4 BRAIN METASTASES/YEAR
-
Protocol number: NRG-BR007-NCT04852887
A PHASE III CLINICAL TRIAL EVALUATING DE-ESCALATION OF BREAST RADIATION FOR CONSERVATIVE TREATMENT OF STAGE I, HORMONE SENSITIVE, HER2-NEGATIVE, ONCOTYPE RECURRENCE SCORE </= 18 BREAST CANCER
-
Protocol number: NRG-BR009-NCT05879926
A Phase III Adjuvant Trial Evaluating the Addition of Adjuvant Chemotherapy to Ovarian Function Suppression plus Endocrine Therapy in Premenopausal Patients with pN0-1, ER-Positive/HER2-Negative Breast Cancer and an Oncotype Recurrence Score ≤ 25 (OFSET)
-
Protocol number: NRG-CC009
PHASE III TRIAL OF STEREOTACTIC RADIOSURGERY (SRS) VERSUS HIPPOCAMPAL-AVOIDANT WHOLE BRAIN RADIOTHERAPY (HA-WBRT) FOR 10 OR FEWER BRAIN METASTASES FROM SMALL CELL LUNG CANCER
-
Protocol number: NRG-GI008-NCT05174169
Colon Adjuvant Chemotherapy Based on Evaluation of Residual Disease
-
Protocol number: NUTIDE-323-NCT05678257
A randomised, open-label, Phase II, dose/schedule optimisation study of NUC-3373/leucovorin/irinotecan plus bevacizumab (NUFIRI-bev) versus 5-FU/leucovorin/irinotecan plus bevacizumab (FOLFIRI-bev) for the treatment of patients with previously treated unresectable metastatic colorectal cancer
-
Protocol number: NUVA-PFC1020
A prospective, multicenter study evaluating the safety and performance of posterior fixation in trauma, reconstructive, and tumor surgery of the occipito-cervico-thoracic spine.
-
Protocol number: NUVA-PFC1020-WEINER
A prospective, multicenter study evaluating the safety and performance of posterior fixation in trauma, reconstructive, and tumor surgery of the occipito-cervico-thoracic spine.
-
Protocol number: NVL-520-01
A Phase 1/2 Study of the Highly Selective ROS1 Inhibitor NVL-520 in Patients with Advanced NSCLC and Other Solid Tumors (ARROS-1)
-
Protocol number: NVL-655-01
A Phase 1/2 Study of the Selective Anaplastic Lymphoma Kinase (ALK) Inhibitor NVL-655 in Patients with Advanced NSCLC and Other Solid Tumors (ALKOVE-1)
-
Protocol number: OCCLUFLEX-NCT05069558
Prospective randomized multi-center controlled clinical investigation comparing PFO outcomes of the Occlutech Flex II PFO Occluder to standard of care PFO occlusion
-
Protocol number: OG-6219-P001
A Phase 2a/b, Randomized, Double-blind, Placebo-controlled, Parallel Group, Multicenter, Clinical Study to Evaluate the Efficacy and Safety of OG-6219 in 3 Dose Levels, in Women 18 to 49 Years of Age with Moderate to Severe Endometriosis-related Pain
-
Protocol number: OHAND-NCT04035005
A PHASE IIIb MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY TO EVALUATE THE EFFICACY AND SAFETY OF OCRELIZUMAB IN ADULTS WITH PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS
-
Protocol number: ONC-392-001
Safety, Pharmacokinetics (PK), and Efficacy of ONC-392 as a Single Agent and in Combination with Pembrolizumab in Advanced Solid Tumors and NSCLC: An Open Label Phase IA/IB Study
-
Protocol number: ORGANOXMETRAPAS-NCT0552632
OrganOx metra® New Enrollment Post-Approval Study Protocol
-
Protocol number: OUTCOMES-PARTIAL-MENISCECTOMY
Outcomes of the Nanoscopic Partial Meniscectomy Versus Standard Arthroscopic Partial Meniscectomy
-
Protocol number: PACES
Anticoagulation for New-Onset Post-Operative Atrial Fibrillation after CABG (PACeS)
-
Protocol number: PACIFIC4-FSQ
A Phase III, Randomized, Placebo-controlled, Double-blind, Multi-center, International Study of Durvalumab With Stereotactic Body Radiation Therapy (SBRT) for the Treatment of Patients With Unresected Stage I/II, Lymph-node Negative Non-small Cell Lung Cancer (PACIFIC-4/RTOG-3515)
-
Protocol number: PAREMA1
PArtial REbreathing for Migraine with Aura 1 A prospective, multi-centre, randomized, double-blind, sham-controlled, parallel-group, group-sequential study to investigate safety and effectiveness of the Rehaler partial rebreathing device, in adults suffering from migraine with aura
-
Protocol number: PE-TRACT
PULMONARY EMBOLISM; THROMBUS REMOVAL WITH CATHETER-DIRECTED THERAPY: THE PE-TRACT TRIAL
-
Protocol number: PERSONA-TINIDIUM
A Post-Market Clinical Follow-up Study to Provide Safety, Performance and Clinical Benefits Data using the Persona Ti-Nidium Total Knee System and Instrumentation
-
Protocol number: PESSARY
Application of Machine Learning Models for Predicting Pessary Fitting Success, Shape, and Size: An Observational Study
-
Protocol number: PGX-ACT
Pharmacogenomics (PGx) Applied to Chronic pain Treatment in primary care (PGx-ACT)
-
Protocol number: PIVOTAL-IDE
Left Atrial Posterior Wall Isolation in Conjunction With Pulmonary Vein Isolation Using the Cryoballoon for Treatment of Persistent Atrial Fibrillation (PIVoTAL) - IDE Trial
-
Protocol number: PL101-HD301
A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Parallel Arm, Multicenter Study Evaluating the Efficacy and Safety of Pridopidine in Patients with Early Stage of Huntington Disease
-
Protocol number: POLYNOVO-NCT04090424
A Pivotal Study to Assess the Safety and Effectiveness of NovoSorb® Biodegradable Temporizing Matrix (BTM) in the Treatment of Severe Burn Skin Injuries.
-
Protocol number: POSITIVELINKS
Pragmatic Efficacy Trial of mHealth to Improve HIV Outcomes in the DC Cohort
-
Protocol number: PR200-104
A Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of PRA023 in Subjects with Systemic Sclerosis Associated with Interstitial Lung Disease (SSc-ILD)
-
Protocol number: PRECISE
Perfusion imaging to identify posterior circulation candidates for thrombectomy
-
Protocol number: PRELUDE-NCT02768402
Percutaneous Mitral Valve Replacement EvaLuation Utilizing IDE Early Feasibility Study (PRELUDE)
-
Protocol number: PREVAIL
Obicetrapib and Cardiovascular Outcomes: A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With Atherosclerotic Cardiovascular Disease (ASCVD) Who are Not Adequately Controlled Despite Maximally Tolerated Lipid-Modifying Therapies
-
Protocol number: PRO2020-0369
A Phase 2B study of selinexor (KPT-330), in combination with carfilzomib, daratumumab or pomalidomide in patients with multiple myeloma relapsing on current therapy
-
Protocol number: PRO2021-1263
Exploratory Study of Fluoroquinolone Resistance for Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation in the Treatment of Multiple Myeloma
-
Protocol number: PROACTIVEHF-NCT04089059
A Prospective, Multi-Center, Open Label, Single Arm Clinical Trial Evaluating the Safety and Efficacy of the Cordella Pulmonary Artery Sensor System in New York Heart Association (NYHA) Class III Heart Failure Patients
-
Protocol number: PROACTIVEHF2-NCT05934487
A Prospective, Multi-Center, Open Label, Randomized Control Clinical Trial Evaluating the Safety and Efficacy of the Cordella™ Pulmonary Artery Sensor System in New York Heart Association (NYHA) Class II Heart Failure Patients
-
Protocol number: PROGEM-NCT05827055
Role of Cholecystokinin Receptor Blockade on the Tumor Microenvironment in Pancreatic Cancer
-
Protocol number: PROGRESS-NCT03777059
A PHASE 3, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, PARALLEL-GROUP STUDY TO EVALUATE THE EFFICACY, SAFETY, AND TOLERABILITY OF ATOGEPANT FOR THE PREVENTION OF CHRONIC MIGRAINE (PROGRESS)
-
Protocol number: PROTECTH2H
Protect the Head to Head: A Safety and Efficacy Assessment of the Emboliner® Embolic Protection Device
-
Protocol number: PRP
Vaginal Injection of Platelet Rich Plasma for the Improvement of Sexual Function (V.I.P. Study)
-
Protocol number: PSR-APV
Aortic, Peripheral & Venous (APV) Product Surveillance Registry Platform Base
-
Protocol number: PTB01
An open, non-comparative, multi-center investigation to evaluate performance and safety of Exufiber Ag+ and Exufiber on partial thickness burns.
-
Protocol number: PTK0796-NTM-20203
A Phase 2, Double-Blind, Randomized, Parallel-Group, Placebo-Controlled, Multi-Center Study to Evaluate the Efficacy, Safety, and Tolerability of Oral Omadacycline in Adult Subjects with Nontuberculous Mycobacterial (NTM) Pulmonary Disease Caused by Mycobacterium abscessus Complex (MABc)
-
Protocol number: PUMA-ALI-4201-NCT06095505
A PHASE 2 STUDY OF ALISERTIB IN PATIENTS WITH EXTENSIVE STAGE SMALL CELL LUNG CANCER
-
Protocol number: RAGE-2021
RAGE inhibition to decrease cancer therapy related cardio toxicity in women with non-metastatic breast cancer
-
Protocol number: REFINE-ALS-NCT04259255
Radicava®/(Edaravone) Findings in Biomarkers From ALS (REFINE-ALS)
-
Protocol number: REGAIN
Regeneration of Acutely Injured Nerves with Temporary Electrical Stimulation (REGAIN)
-
Protocol number: REN007NCT06021457
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of RBT-1 on Reducing the Risk of Post-Operative Complications in Subjects Undergoing Cardiac Surgery
-
Protocol number: RESTORE-NCT02586623
RESTORE: A Clinical Study of Patients With Symptomatic Neurogenic Orthostatic Hypotension to Assess Sustained Effects of Droxidopa Therapy
-
Protocol number: RIN-PF-301
A Randomized, Double-blind, Placebo-controlled, Phase 3 Study of the Efficacy and Safety of Inhaled Treprostinil in Subjects with Idiopathic Pulmonary Fibrosis
-
Protocol number: ROADSTER3-NCT05365490
POST-APPROVAL STUDY of TRANSCAROTID ARTERY REVASCULARIZATION in STANDARD RISK PATIENTS with SIGNIFICANT CAROTID ARTERY DISEASE The ROADSTER 3 Study
-
Protocol number: ROCHEBP43176
A PHASE 1B, ADAPTIVE, MULTI-CENTER, RANDOMIZED, DOUBLE BLIND, PLACEBO-CONTROLLED, PARALLEL DESIGN STUDY TO INVESTIGATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS AND PHARMACODYNAMICS OF RO7486967 IN PARTICIPANTS WITH EARLY IDIOPATHIC PARKINSON S DISEASE
-
Protocol number: RP-2021-IST
A multicenter observational data registry for outcomes of inappropriate sinus tachycardia and postural orthostatic tachycardia syndrome treatment.
-
Protocol number: RPNI
Regenerative Peripheral Nerve Interfaces to Treat Painful Digit and Hand Neuromas After Amputation: A Randomized, Prospective Study
-
Protocol number: RRTCR1-LGGAIM1
Intravesical Lactobacillus for Urinary Symptoms Among People With NLUTD Who Use Indwelling Catheters AIM 1
-
Protocol number: RRTCR1-LGGAIM2
Intravesical Lactobacillus for Urinary Symptoms Among People With NLUTD Who Use Indwelling Catheters AIM 2
-
Protocol number: RT234-PAH-CL202
A Phase 2b, Open-label, Single Dose Study to Evaluated the Safety and Efficacy of RT234 on Exercise Parameters Assessed by Cardiopulmonary Exercise Testing (CPET) in Subjects With Pulmonary Arterial Hypertension (PAH)
-
Protocol number: RTOG1308-LUNGCANC-NCT01993810
Comparing Photon Therapy To Proton Therapy To Treat Patients With Lung Cancer
-
Protocol number: RYZ101-301
Phase 1b/3 global, randomized, controlled, open-label trial comparing treatment with RYZ101 to standard of care (SoC) therapy in subjects with inoperable, advanced, somatostatin receptor expressing (SSTR+), well-differentiated gastro-enteropancreatic neuroendocrine tumors (GEP-NETs) that have progressed following prior 177Lu labelled somatostatin analogue (177Lu-SSA) therapy (ACTION-1)
-
Protocol number: S1925-NCT04269902
Randomized, Phase III Study of Early Intervention with Venetoclax and Obinutuzumab Versus Delayed Therapy with Venetoclax and Obinutuzumab in Newly Diagnosed Asymptomatic High-Risk Patients with Chronic Lymphocytic Leukemia / Small Lymphocytic Lymphoma (CLL/SLL): EVOLVE CLL/SLL Study.
-
Protocol number: S1931-PROBE
Phase III Trial of Immunotherapy-Based Combination Therapy With or Without Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma (PROBE Trial)
-
Protocol number: S2015
Melanoma Margins Trial (MelMarT): A Phase III, multi-centre, multi-national randomised control trial investigating 1cm v 2cm wide excision margins for primary cutaneous melanoma
-
Protocol number: S2302-NCT05633602
Pragmatica-Lung: A Prospective Randomized Study of Ramucirumab (LY3009806; NSC 749128) Plus Pembrolizumab (MK-3475; NSC 776864) Versus Standard of Care for Participants Previously Treated With Immunotherapy for Stage IV or Recurrent Non-Small Cell Lung Cancer
-
Protocol number: SAFEKIDNEY
Safety, tolerability and efficacy of AntiBKV as treatment of BKV infection in kidney transplant recipients, a randomized phase II/III study, double-blind and placebo-controlled
-
Protocol number: SAGE324-ETD-202
A Phase 2, Double-Blind, Randomized, Placebo-Controlled, Dose-Response Study of SAGE-324 for the Treatment of Essential Tremor
-
Protocol number: SANOFIACT16970
A Phase 2, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of SAR443820 in adult participants with amyotrophic lateral sclerosis, followed by an open-label extension
-
Protocol number: SAVISCOUT
SAVI SCOUT® System for Excision of nonpalpable breast lesions: Single institutional study regarding experiences, lessons learned & comparisons to needle localization
-
Protocol number: SCONE
Transformation of Dormant Spinal Networks to Mitigate Symptoms of Neurogenic Bladder ( SCONE Clinical Study )
-
Protocol number: SECURE
A ProSpective, MulticEnter, Concurrently Controlled Clinical Study of Chondro-Gide® ArticUlar Cartilage CoveR for the Treatment of Large Chondral Lesions in the KnEe (SECURE)
-
Protocol number: SELUTION-SLR-NCT04280029
SELUTION SLR™ 014 ISR: A Prospective Randomized Single Blind Multicenter Study to Assess the Safety and Effectiveness of the SELUTION SLR™ 014 Drug Eluting Balloon in the Treatment of Subjects with In-stent Restenosis
-
Protocol number: SELUTION4BTK
A Prospective Randomized Multicenter Single Blinded Study to Assess the Safety and Effectiveness of the SELUTION SLR™ 014 Drug Eluting Balloon in the Treatment of CLTI Patients with BTK Artery Lesions
-
Protocol number: SELUTION4SFA
A Prospective Randomized Multicenter Single Blinded Study to Assess the Safety and Efficacy of the SELUTION SLR™ 018 Drug Eluting Balloon in the Treatment of Subjects with Femoropopliteal Artery Lesions
-
Protocol number: SER-155-001-NCT04995653
A Phase 1b Study to Evaluate Safety, Tolerability, Pharmacokinetics, and Efficacy of SER-155 in Adults Undergoing Hematopoietic Stem Cell Transplantation (HSCT) to Reduce the Risk of Infection and Graft vs. Host Disease (GvHD)
-
Protocol number: SISTER
Social Interventions for Support during Treatment for Endometrial Cancer and Recurrence (SISTER): a multi-site randomized controlled trial
-
Protocol number: SJS-TENS
Comprehensive Genomic Analysis of Adverse Vaccine and Cutaneous Adverse Drug Reactions
-
Protocol number: SMA-CHART-REVIEW-NCT99999999
Emerging Real-World Use of Nusinersen in Adult Patients with Spinal Muscular Atrophy (SMA) in the US: A Multi-Site Chart Review Study
-
Protocol number: SMART-TRIAL
SMall Annuli Randomized To Evolut or SAPIEN Trial (SMART Trial)
-
Protocol number: SOLAR
Transvaginal Photobiomodulation for the Treatment of Dyspareunia in Endometriosis Patients: A Randomized Controlled Trial
-
Protocol number: SOS-AMI
Multi-center, double-blind, randomized, placebo-controlled, parallel-group study to evaluate the efficacy and safety of self-administered subcutaneous selatogrel for prevention of all-cause death and treatment of acute myocardial infarction in subjects with a recent history of acute myocardial infarction
-
Protocol number: SPLIT
Prospective Observational Study to Assess Outcomes in Pediatric Liver Transplant Recipients
-
Protocol number: SPR720-202
A Randomized, Double-Blinded, Placebo-Controlled, Multicenter, Phase 2, Dose-Ranging Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of SPR720 Compared with Placebo for the Treatment of Patients with Mycobacterium avium Complex (MAC) Pulmonary Disease
-
Protocol number: SPYRALAFFIRM
The SPYRAL AFFIRM Global Clinical Study of Renal Denervation with the Symplicity Spyral Renal Denervation System in Subjects with Uncontrolled Hypertension (SPYRAL AFFIRM)
-
Protocol number: STAR-221
A Randomized, Open-Label, Multicenter Phase 3 Trial of Domvanalimab, Zimberelimab,and Chemotherapy Versus Nivolumab and Chemotherapy in Participants with Previously-Untreated Locally Advanced Unresectable or Metastatic Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma
-
Protocol number: STEM-PD
Non-Invasive Brainstem Modulation for the Treatment of Non-Motor Symptoms in Parkinson’s Disease: A Randomized Controlled Trial (RCT) and an Open Label Extension (OLE) Study
-
Protocol number: STEP-1
A Randomized, Multi-Center, Pivotal Efficacy and Safety Study Evaluating the EndoBarrier® System for Glycemic Improvement in Patients with Inadequately Controlled Type 2 Diabetes and Obesity, the STEP-1 Study
-
Protocol number: STRO-002-GM3
A Phase 2 Open-label Study Evaluating the Efficacy and Safety of Luveltamab Tazevibulin (STRO-002) in Women with Relapsed Platinum-resistant Epithelial Ovarian Cancer (including Fallopian Tube or Primary Peritoneal Cancers) expressing Folate Receptor alpha (FOLR1)
-
Protocol number: STUDY00000017
A randomized, double blind, placebo-controlled study to evaluate the impact of Bosutinib on safety, tolerability, biomarkers and clinical outcomes in Dementia with Lewy Bodies (DLB)
-
Protocol number: STUDY00000122
A randomized, double blind, placebo-controlled study to evaluate the impact of Nilotinib treatment on safety, tolerability, pharmacokinetics and biomarkers in Dementia with Lewy Bodies (DLB)
-
Protocol number: STUDY00000266
A randomized, double blind, placebo-controlled study to evaluate the impact of K0706 on safety, tolerability, pharmacokinetics and pharmacodynamics and clinical outcomes in Dementia with Lewy Bodies (DLB).
-
Protocol number: STUDY00000307
Identifying early neuroimaging biomarkers using diffusivity measures and functional connectivity to predict neurodevelopmental outcomes in neonates with hypoxic ischemic injury
-
Protocol number: STUDY00000501
Chronic Stress, QOL, and Physical Activity among Breast Cancer Survivors
-
Protocol number: STUDY00001007
Peer Support For Young Adult Women With High Breast Cancer Risk
-
Protocol number: STUDY00001094
Evaluating Screening Breast MRI Utilization and Optimal Screening Intervals at a Tertiary Care Center
-
Protocol number: STUDY00001137
Parent Communication Study IV: Improving Genetic Counseling for BRCA+ Mothers
-
Protocol number: STUDY00001487-TBCRC047
Innovative Combination Immunotherapy for Metastatic Triple Negative Breast Cancer (TNBC): A Multicenter, Multi-Arm Translational Breast Cancer Research Consortium Study
-
Protocol number: STUDY00001920
Programa de ARBOLES Familiares: Assessing Risk of Breast Cancer through Outreach to Latinas with Education and Support
-
Protocol number: STUDY00002424
Investigating Exposure to Endocrine Disrupting Chemicals and Poor Sleep in Breast Cancer Survivors
-
Protocol number: STUDY00002789
CONTIGO - Informing Latinas About HBOC Risk: a Randomized Controlled Trial
-
Protocol number: STUDY00003077-NCT04520776
A multicenter, prospective, randomized, clinical trial comparing the safety and effectiveness of the BAGUERA® C Cervical Disc Prosthesis to the Mobi-C® Cervical Disc for the treatment of patients with symptomatic cervical disc disease at a single level.
-
Protocol number: STUDY00003267
Families SHARE: Development and evaluation of a family health history education toolkit
-
Protocol number: STUDY00003277
A mutlilevel intervention to address disparities in lung cancer screening
-
Protocol number: STUDY00003285
Use of MR Spectroscopy (MRS) to Differentiate Vertebral Metastases from Atypical Vertebral Hemangiomas
-
Protocol number: STUDY00003342
Longitudinal investigation of sociocultural and behavioral influences on symptom management, biological response, and functioning between Chinese and White breast cancer survivors
-
Protocol number: STUDY00003415
Supporting Shared Decision Making in Metastatic Breast Cancer
-
Protocol number: STUDY00003539
Improving Communication and Adherence in Black Breast Cancer Survivors
-
Protocol number: STUDY00003673
Developing a Multilevel Intervention for Screening Breast MRI
-
Protocol number: STUDY00003772
Understanding HPV Vaccination Behavior to Prevent Cancer
-
Protocol number: STUDY00003788
Identifying early neuroimaging biomarkers using diffusivity measures and functional connectivity to predict neurodevelopmental outcomes in neonates with prenatal opioid exposure
-
Protocol number: STUDY00004071
An advanced functional MRI study of frontostriatal injury in adults with HIV
-
Protocol number: STUDY00004397
Scaling Social Determinants of Health Screening, Social Support and Anti-Racism Training to Reduce Inequities in Minority Cancer Survivor Health and Wellbeing in Washington, DC
-
Protocol number: STUDY00004595
Pilot Testing of Self-Acupressure Intervention to Improve Function and Symptom Outcomes among Black and Latina Breast Cancer Survivors
-
Protocol number: STUDY00004914
Providing Tobacco Treatment to Patients Undergoing Lung Cancer Screening at MedStar Health: Pilot Study
-
Protocol number: STUDY00005081
Ecological Momentary Assessment of Quality of Life in Metastatic Breast Cancer
-
Protocol number: STUDY00005085
Examining the relationship between social and molecular risk factors for Black patients with endometrial cancer
-
Protocol number: STUDY00005278
Reducing barriers to COVID-19 vaccinations in Black/African American and Hispanic/Latin American breast cancer survivors
-
Protocol number: STUDY00005285
Development of an informational support intervention for patients with differentiated thyroid cancer after radioactive iodine treatment
-
Protocol number: STUDY00005453-NCT05558982
Phase II Trial of BXCL701 and Pembrolizumab in Patients with Metastatic Pancreatic Ductal Adenocarcinoma
-
Protocol number: STUDY00005472-ISTPROTOCOL
Istradefylline Effect on Parkinson’s Disease Tremor, Motor Symptoms and Non-motor Symptoms
-
Protocol number: STUDY00005505
A double blind, randomized, placebo-controlled trial evaluating the efficacy and safety of BI 1015550 over at least 52 weeks in patients with Idiopathic Pulmonary Fibrosis (IPF)
-
Protocol number: STUDY00005554
Peripheral and central contributions to auditory temporal processing deficits and speech understanding in older cochlear implantees
-
Protocol number: STUDY00006070
Providing Tobacco Treatment to Patients Undergoing Lung Cancer Screening at MedStar Health: A Randomized Trial
-
Protocol number: STUDY00006368
Implementing a Toolkit to Improve Family Communication about Inherited Cancer Syndromes
-
Protocol number: STUDY00006573
Glucose-Guided Eating (GGE) to reduce chronic disease risk: a pilot study
-
Protocol number: STUDY00006715-GENETICS
Understanding Communication Practices and Support Needs around Genetic Testing among Black Breast Cancer Patients Families
-
Protocol number: STUDY00006772
Pridopidine Expanded Access Program (EAP) for Huntington Disease
-
Protocol number: SUMMIT-NCT03433274
Clinical Trial to Evaluate the Safety and Effectiveness of Using the Tendyne Mitral Valve System for the Treatment of Symptomatic Mitral Regurgitation
-
Protocol number: SUN-PRO00038942
Clinical Trial for Surgery of the Ulnar Nerve (SUN) at the Elbow
-
Protocol number: SURPLUS
Surgical Resection of Prosthetic Valve Leaflets Under Direct Vision (SURPLUS) Registry
-
Protocol number: SYNEX
Efficacy of SynEx wound rinse in civilian surrogates of combat injury wounds.
-
Protocol number: TACT-STUDY00005956
Qualitative Study of Physical Activity of Children Undergoing Cancer Therapy
-
Protocol number: TAKEDA
A Phase 3, Prospective, Randomized, Open-label, Adaptive Group Sequential, Multicenter Trial with Blinded Endpoint Assessment to Evaluate the Efficacy and Safety of PROTHROMPLEX TOTAL for the Reversal of Direct Oral Factor Xa Inhibitor-induced Anticoagulation in Patients Requiring Urgent Surgery/Invasive Procedure
-
Protocol number: TARGET-ALS
Target ALS Biomarker Study: Longitudinal Biofluids, Clinical Measures, and At-Home Measures
-
Protocol number: TARGET-HCC-NCT02954094
A 5-year Longitudinal Observational Study of the Natural History and Management of Patients with Hepatocellular Carcinoma
-
Protocol number: TARGET-LD-WHC
An Observational Study of Patients with Chronic Liver Disease
-
Protocol number: TARGET-NASH-NCT02815891
A 5-year Longitudinal Observational Study of Patients with Nonalcoholic Fatty Liver or Nonalcoholic Steatohepatitis
-
Protocol number: TBCRC-052
MARGetuximab Or Trastuzumab (MARGOT): A Phase II Study Comparing Neoadjuvant Paclitaxel/Margetuximab/Pertuzumab to Paclitaxel/Trastuzumab/Pertuzumab in Patients With Stage II-III HER2-positive Breast Cancer
-
Protocol number: TBCRC046-NCT04841148
A Phase II Trial of Avelumab or Hydroxychloroquine with or without Palbociclib to Eliminate Dormant Breast Cancer ( PALAVY )
-
Protocol number: TBCRC062-NCT05721248
The STOP-HER2 Trial: A Phase 2 Study of Stopping Trastuzumab - Outcomes in Patients with HER2+ Metastatic Breast Cancer
-
Protocol number: TEASER-NCT02540109
Targeted Transcranial Electrotherapy to Accelerate Stroke Rehabilitation-Exploratory Trial on Aphasia
-
Protocol number: TELLTALE
NHLBI Transmural Electrosurgery LeafLet Traversal And Laceration Evaluation (TELLTALE) BASILICA-TAVR Trial
-
Protocol number: TH-IBA-CTR-1003
Theratechnologies Inc. - A Prospective and Retrospective Observational Study of Multidrug-Resistant Patient Outcomes with and without Ibalizumab in a Real-World Setting: United States (PROMISE-US)
-
Protocol number: THUMB-CMC
Prospective Longitudinal Study of Non-Operative and Operative Thumb CMC Arthritis Interventions
-
Protocol number: THUMB-CMC-MEANS
Evaluating a Hand Therapy Digital Web-based Application for Thumb Carpometacarpal Arthritis
-
Protocol number: TIGER-001
A Global Post Market Evaluation of Terumo Aortic Endovascular Grafts
-
Protocol number: TJ003234COV201
A Phase 1b/2, Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Evaluate the Safety and Efficacy of TJ003234 in Subjects with Severe Coronavirus Disease 2019 (COVID-19)
-
Protocol number: TRAC-AFOUTCOMES
Tracking Results of Ablations to Combat AF Registry (Formerly the Maze IV Registry)
-
Protocol number: TRACAF-NCT000000000
Tracking Results of Ablations to Combat AF Registry
-
Protocol number: TRANSPORT2-NCT03826030
TRANScranial direct current stimulation for POst-stroke motor Recovery - a phase II sTudy (TRANSPORT 2)
-
Protocol number: TREATMS-NCT03500328
TRADITIONAL VERSUS EARLY AGGRESSIVE THERAPY FOR MULTIPLE SCLEROSIS (TREAT-MS) TRIAL
-
Protocol number: TREO-PAS
Post-Approval Study of the TREO Abdominal Stent- Graft System (P190015) in Patients with Infrarenal Abdominal Aortic and Aorto-iliac Aneurysms
-
Protocol number: TRILUMINATE-NCT03904147
Clinical TRIal to EvaLUate Cardiovascular OutcoMes IN PAtients Treated with the Tricuspid ValvE Repair System Pivotal
-
Protocol number: TRIOMPHE-NCT04471909
A Multi-Arm, Multi-Center, Non-Randomized, Prospective, Clinical Study to Evaluate the Safety and Effectiveness of the NEXUS Aortic Arch Stent Graft System in Treating Thoracic Aortic Lesions Involving the Aortic Arch
-
Protocol number: TRIUMPH-NCT99999999
Protocol I5Q-MC-B004: preventive TReatment of mIgraine: oUtcoMes for Patients in real-world Healthcare systems (TRIUMPH)
-
Protocol number: TTNS-NCT04350359
Transcutaneous Tibial Nerve Stimulation for Spinal Cord Injury Neurogenic Bladder
-
Protocol number: TUMORCOLLAGEN
Tumor Associated Collagen Signature in Hepatocellular Carcinoma
-
Protocol number: TVD-101-001H-NCT05440708
A Phase 1b/2 Multicenter, Open-label Study to Evaluate the Safety and Efficacy of TTI-101 as Monotherapy and in Combination in Participants with Locally Advanced or Metastatic, and Unresectable Hepatocellular Carcinoma
-
Protocol number: TYR-219-01-NCT04474470
A Phase 1/2 Study with Open-Label, Dose Escalation Phase Followed by Single-Arm Expansion at the Maximum Tolerated Dose to Assess the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of NT219 Injection alone and in Combination with ERBITUX® (Cetuximab) in Adults with Advanced solid tumors and Head and Neck cancer
-
Protocol number: U-C-TPD-2022-01-GSH
Randomized, Controlled, Open-Label, Parallel Group, Multi-Center, Prospective Phase 4 Study Comparing the Efficacy of Transforming Powder Dressing to NPIAP Recommended Standard of Care Therapies in Stage 2, 3 and 4 Pressure Injuries
-
Protocol number: U-C-TPD-2022-01-NRH
Randomized, Controlled, Open-Label, Parallel Group, Multi-Center, Prospective Phase 4 Study Comparing the Efficacy of Transforming Powder Dressing to NPIAP Recommended Standard of Care Therapies in Stage 2, 3 and 4 Pressure Injuries
-
Protocol number: U-C-TPD-2022-01-WHC
Randomized, Controlled, Open-Label, Parallel Group, Multi-Center, Prospective Phase 4 Study Comparing the Efficacy of Transforming Powder Dressing to NPIAP Recommended Standard of Care Therapies in Stage 2, 3 and 4 Pressure Injuries
-
Protocol number: U31402-A-U103
A PHASE 1 OPEN-LABEL STUDY OF PATRITUMAB DERUXTECAN (U3-1402) IN COMBINATION WITH OSIMERTINIB IN SUBJECTS WITH LOCALLY ADVANCED OR METASTATIC EGFR-MUTATED NONSMALL CELL LUNG CANCER (NSCLC)
-
Protocol number: UCL-CHDI-1
HDClarity: a multi-site cerebrospinal fluid collection initiative to facilitate therapeutic development for Huntington s disease
-
Protocol number: ULURU-BURN
Prospective Randomized Open Label Multicenter Phase IV Clinical Trial to Compare Transforming Powder Dressing (TPD) to Current Standard of Care (SOC) Dressing Therapies in Acute Partial Thickness Burn Wounds
-
Protocol number: URCC-21038-DIRECT-NCT05364086
An Observational Research Study for Cancer Patients on Immune Checkpoint Inhibitors, DiRECT Study (DiRECT)
-
Protocol number: URCC18110CD-ENABLE-NCT04062552
Learning Collaborative Vs Technical Assistance in Delivering a Palliative Care Program to Patients With Advanced Cancer and Their Caregivers
-
Protocol number: VASOMUNE
A Randomized, Double-blind, Placebo-controlled, Phase 2a Multiple Ascending Dose Study to Examine the Safety, Tolerability and Efficacy of AV-001 in Patients Hospitalized with Confirmed Severe COVID-19 Disease
-
Protocol number: VERIFY-NCT05338697
Validation of Early Prognostic Data for Recovery Outcome After Stroke for Future, Higher Yield Trials (VERIFY)
-
Protocol number: VERISMO
AN OBSERVATIONAL STUDY OF OCRELIZUMABTREATED PATIENTS WITH MULTIPLE SCLEROSIS TO DETERMINE THE INCIDENCE AND MORTALITY RATES OF BREAST CANCER AND ALL MALIGNANCIES (VERISMO STUDY)
-
Protocol number: VIB0551-P3-S1
A RANDOMIZED, DOUBLE-BLIND, MULTICENTER, PLACEBO-CONTROLLED PHASE 3 STUDY WITH OPEN-LABEL PERIOD TO EVALUATE THE EFFICACY AND SAFETY OF INEBILIZUMAB IN ADULTS WITH MYASTHENIA GRAVIS
-
Protocol number: VICTORION-INCEPTION
A randomized, controlled, multicenter, open-label trial comparing a hospital post-discharge care pathway involving aggressive LDL-C management that includes inclisiran with usual care versus usual care alone in patients with a recent acute coronary syndrome(VICTORION-INCEPTION)
-
Protocol number: VICTORION-PLAQUE
A multi-center, randomized, double-blind, placebo-controlled, parallel-group phase IIIb study evaluating the effect of inclisiran on atherosclerotic plaque progression assessed by coronary computed tomography angiography (CCTA) in participants with a diagnosis of non-obstructive coronary artery disease without previous cardiovascular events.
-
Protocol number: VIIV
A multi- site observational study to assess safety and effectiveness of prenatal exposure to Dolutegravir in HIV positive pregnant women
-
Protocol number: VLU-ORGANOGENESIS
A Prospective, Multicenter, Randomized, Controlled Clinical Study Of Affinity plus Standard of Care (SOC) compared to SOC alone In The Management Of Venous Leg Ulcers (VLUs)
-
Protocol number: VLX-1005-003-MEDSTAR
A RANDOMIZED, DOUBLE-BLIND, PHASE 2 PILOT STUDY OF VLX-1005 VERSUS PLACEBO IN PARTICIPANTS WITH SUSPECTED HEPARIN INDUCED THROMBOCYTOPENIA TREATED WITH BACKGROUND STANDARD OF CARE
-
Protocol number: VLX-301
A Randomized Double-Blind Placebo-Controlled Study to Evaluate the Efficacy and Safety of Volixibat in the Treatment of Cholestatic Pruritus in Patients with Primary Sclerosing.Cholangitis (VISTAS.
-
Protocol number: VLX-601
A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Volixibat in the Treatment of Cholestatic Pruritus in Patients with Primary Biliary Cholangitis (VANTAGE)
-
Protocol number: VNS-21-07-NCT05489588
Evaluation of the GORE® VIAFORT Vascular Stent for Treatment of Symptomatic Iliofemoral Venous Obstruction
-
Protocol number: VS-6766-203
A Phase 1/2 Study of VS-6766 in Combination with Sotorasib in Patients with KRAS G12C mutant Non-Small Cell Lung Cancer (NSCLC) (RAMP 203)
-
Protocol number: VT-305
A PHASE 3, PROSPECTIVE, RANDOMIZED, MULTICENTER, SINGLE-BLIND, CONTROLLED STUDY EVALUATING ARTERIOVENOUS FISTULA OUTCOMES WITH AND WITHOUT A PERIVASCULAR SIROLIMUS-ELUTING COLLAGEN IMPLANT (THE ACCESS 2 TRIAL)
-
Protocol number: VULVIE
A randomized controlled trial of Vulvar fractionated CO2-Laser therapy with and without concomitant topical clobetasol propionate 0.05% ointment for treatment of Vulvar lichen sclerosus
-
Protocol number: VX15/2503-11
SEMA4D Blockade Safety and Brain Metabolic Activity in Alzheimer s Disease (AD): A Phase 1b/2a, Multi-center, Randomized, Double-Blind, Placebo-Controlled Safety and Biomarker Study of Pepinemab Anti-SEMA4D Antibody in early-AD
-
Protocol number: WATS-NCT02988934
The WATS3-D (Wide Area Transepithelial Sample Biopsy with 3-Dimensional Computer-Assisted Analysis) U.S. Registry
-
Protocol number: WF-1805CD-HN-STAR-NCT04208490
Implementation and Effectiveness Trial of HN-STAR
-
Protocol number: WF-1806-NCT03998202
Myopenia and Mechanisms of Chemotherapy Toxicity in Older Adults With Colorectal Cancer (M&M) (NCORP)
-
Protocol number: WN42171
AN OPEN-LABEL, MULTICENTER, ROLLOVER STUDY TO EVALUATE THE SAFETY, TOLERABILITY, AND EFFICACY OF LONG-TERM GANTENERUMAB ADMINISTRATION IN PARTICIPANTS WITH ALZHEIMER'S DISEASE
-
Protocol number: WN43194
A PHASE III, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO EVALUATE THE EFFICACY, SAFETY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF SATRALIZUMAB AS MONOTHERAPY OR IN ADDITION TO BASELINE THERAPY IN PATIENTS WITH MYELIN OLIGODENDROCYTE GLYCOPROTEIN ANTIBODY-ASSOCIATED DISEASE (MOGAD)
-
Protocol number: WO44263-INAVO122
A PHASE III, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY EVALUATING THE EFFICACY AND SAFETY OF INAVOLISIB IN COMBINATION WITH PHESGO VERSUS PLACEBO IN COMBINATION WITH PHESGO AS MAINTENANCE THERAPY AFTER FIRST LINE INDUCTION THERAPY IN PARTICIPANTS WITH PIK3CA MUTATED HER2 POSITIVE LOCALLY ADVANCED OR METASTATIC BREAST CANCER
-
Protocol number: XB002-101
A Dose-Escalation and Expansion Study of the Safety and Pharmacokinetics of XB002 as Single-Agent and Combination Therapy in Subjects with Inoperable Locally Advanced or Metastatic Solid Tumors
All clinical trials
Our trials are carefully planned research studies that have led to important discoveries such as new drugs, procedures, and diagnostic approaches that make our lives better.
Volunteering in our trial offers potential benefits, including:
-
The opportunity to become involved in the testing of a new drug that may have potential to improve your condition
-
Close contact with the study team for management of your disease
-
Contributing to medical science, which may help others now and in the future
Frequently asked questions
-
Q: Why volunteer?
A: Does volunteering for a clinical trial make sense for you? The following information will help you to decide.
-
Q: What are clinical trials and why are they important?
A: Clinical trials are carefully planned research studies that have led to important discoveries such as new drugs, devices, procedures, biologics, and diagnostic approaches. These new discoveries make our lives better such as new medicines to treat cancer, diabetes, and other diseases. Some other words for clinical trials are research, survey, or experiment.
Know what you are getting into and don’t be afraid to ask questions. A clinical trial may or may not help you personally. The results from the study could help others who have a health problem. Taking part in research is voluntary. It is your decision!
-
Q: Why do we need clinical trials?
A: A series of clinical trials for each possible treatment must be done before the Food and Drug Administration (FDA) will approve a drug, procedure, or device. Many of the trials done at MedStar Health Research Institute are trials testing possible drugs or devices. A drug must be shown to be safe and useful for public use before the FDA can approve it.
From the lab bench to the drug store, developing a new drug is a long and expensive process. It is estimated to take about 10 years and $800 million to bring one new drug to market (R & D Directions, January 2002).
-
Q: Are clinical trials safe?
A: Protections have been put in place to safeguard your rights, safety, and privacy in research. Many layers of oversight include an institutional review board (ethical review board), the Food and Drug Administration (FDA), and periodic monitoring of study data by independent experts, patient advocates, community advisory board members, and community partners.
-
Screening trials test the best way to detect certain diseases or health conditions
-
Diagnostic trials determine better tests or procedures for diagnosing a particular disease or condition
-
Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery
-
Quality of life trials explore and measure ways to improve the comfort and quality of life of people with a chronic illness
-
-
Q: Who can participate in a clinical trial?
A: People with the condition being studied or healthy people can volunteer to take part in a clinical trial. Each study has specific entry requirements such as age or a medical condition necessary for participation.
The doctor in charge of the study must review the volunteers' medical history to determine if he/she is eligible to participate based on strict study conditions called entry criteria.
-
Q: Who is in charge of a clinical trial?
A: A medical doctor is usually responsible for carrying out the study according to the study protocol. Before a clinical trial can begin to enroll volunteers, an Institutional Review Board (IRB) must approve it. The IRB is a committee made up of healthcare experts that review each study to protect patient rights and ensure the safety of research volunteers. The IRB also ensures the research is scientifically sound and is involved in the review of safety issues throughout the study.
-
Q: What is informed consent?
A: One of the documents the IRB must review and approve is called an Informed Consent Form. This is provided to volunteers who are interested in participating in a clinical trial program. The informed consent includes everything a volunteer needs to know about the study so that he/she can make an informed decision as to whether or not to participate. The process allows volunteers to ask questions. Volunteers are encouraged to take their time in making a decision before signing the consent and joining the study. You and your family should feel completely comfortable with your decision to participate before you sign the consent.
-
Q: What are the benefits to being in a clinical trial?
A: Once enrolled in a clinical trial, many volunteers find there are a number of potential benefits to participation. These can include:
-
The opportunity to become involved in the testing of a new drug that may have potential to improve your condition.
-
Close contact with the study team for management of your disease.
-
Contributing to medical science, which may help others now and in the future.
However, volunteers understand that there may not be any benefit to participating in a study.
-
-
Q: Why do we need diversity in a research?
A: Race, ethnicity, age, and other factors can affect how people respond to medicine.
-
Q: Why do many still feel uneasy about being part of a clinical trial?
A: The Tuskegee Syphilis Study and the cancer cells taken from Henrietta Lacks without her knowledge are two reasons for the continued distrust with the African American community. Today, there is low participation in clinical trials from African Americans. We may not know if certain treatments will be effective in this population.
-
Q: Will my health insurance pay for a clinical trial?
A: Federal law requires insurance companies to cover routine costs, which are things you need regardless of a clinical trial. If the study sponsor requires extra testing, they will typically cover the cost.
-
Q: Can I drop out of a clinical trial and still receive quality care?
A: You are in control. If you decide you do not want to participate anymore, you can withdraw at any time. You will receive the same high-quality care at MedStar Health. Your clinical team may ask to continue monitoring you for a period of time to assess effects of the treatment you have already received.
-
Q: Are clinical trials considered a last-ditch effort for patients?

A: Not true at all! Clinical trials allow access to treatments that are up and coming but not yet available to the general population.
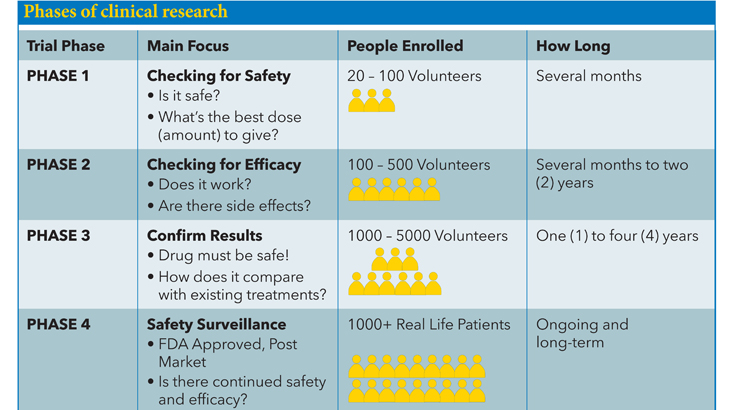
-
Q: Interested in participating in a clinical trial by MedStar Health?
A: Email JoinResearch@MedStar.net or call 833-998-0900 (toll-free) to learn more and ask about our open studies.










